Nucleosome dynamics as modular systems that integrate DNA damage and repair
- PMID: 24003210
- PMCID: PMC3753706
- DOI: 10.1101/cshperspect.a012658
Nucleosome dynamics as modular systems that integrate DNA damage and repair
Abstract
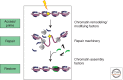
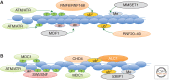
By some estimates, a eukaryotic cell must repair up to 10,000 DNA lesions per cell cycle to counteract endogenous sources of DNA damage. Exposure to environmental toxins, UV sources, or other radiations only increases this enormous number. Failure to repair such lesions can lead to a deleterious mutation rate, genomic instability, or cell death. The timely and efficient repair of eukaryotic DNA damage is further complicated by the realization that DNA lesions must be detected and repaired in the context of chromatin with its complex organization within the nucleus. Numerous studies have shown that chromatin packaging can inhibit nearly all repair pathways, and recent work has defined specific mechanisms that facilitate DNA repair within the chromatin context. In this review, we provide a broad overview of chromatin regulatory mechanisms, mainly at the nucleosomal level, and then focus on recent work that elucidates the role of chromatin structure in regulating the timely and efficient repair of DNA double-strand breaks (DSBs).
Figures
References
-
- Abraham RT 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev 15: 2177–2196 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
