Arginine residues on the opposite side of the active site stimulate the catalysis of ribosome depurination by ricin A chain by interacting with the P-protein stalk
- PMID: 24003229
- PMCID: PMC3798493
- DOI: 10.1074/jbc.M113.510966
Arginine residues on the opposite side of the active site stimulate the catalysis of ribosome depurination by ricin A chain by interacting with the P-protein stalk
Abstract
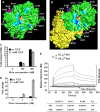
Ricin inhibits protein synthesis by depurinating the α-sarcin/ricin loop (SRL). Ricin holotoxin does not inhibit translation unless the disulfide bond between the A (RTA) and B (RTB) subunits is reduced. Ricin holotoxin did not bind ribosomes or depurinate them but could depurinate free RNA. When RTA is separated from RTB, arginine residues located at the interface are exposed to the solvent. Because this positively charged region, but not the active site, is blocked by RTB, we mutated arginine residues at or near the interface of RTB to determine if they are critical for ribosome binding. These variants were structurally similar to wild type RTA but could not bind ribosomes. Their K(m) values and catalytic rates (k(cat)) for an SRL mimic RNA were similar to those of wild type, indicating that their activity was not altered. However, they showed an up to 5-fold increase in K(m) and up to 38-fold decrease in kcat toward ribosomes. These results suggest that the stalk binding stimulates the catalysis of ribosome depurination by RTA. The mutated arginines have side chains behind the active site cleft, indicating that the ribosome binding surface of RTA is on the opposite side of the surface that interacts with the SRL. We propose that stalk binding stimulates the catalysis of ribosome depurination by orienting the active site of RTA toward the SRL and thereby allows docking of the target adenine into the active site. This model may apply to the translation factors that interact with the stalk.
Keywords: Protein Synthesis; RIP; Ribosomal RNA (rRNA); Ribosome-inactivating Protein; Ribosomes; Ricin; Toxins.
Figures




Similar articles
-
Ricin uses arginine 235 as an anchor residue to bind to P-proteins of the ribosomal stalk.Sci Rep. 2017 Feb 23;7:42912. doi: 10.1038/srep42912. Sci Rep. 2017. PMID: 28230053 Free PMC article.
-
Leucine 232 and hydrophobic residues at the ribosomal P stalk binding site are critical for biological activity of ricin.Biosci Rep. 2019 Oct 30;39(10):BSR20192022. doi: 10.1042/BSR20192022. Biosci Rep. 2019. PMID: 31548364 Free PMC article.
-
Peptide Mimics of the Ribosomal P Stalk Inhibit the Activity of Ricin A Chain by Preventing Ribosome Binding.Toxins (Basel). 2018 Sep 13;10(9):371. doi: 10.3390/toxins10090371. Toxins (Basel). 2018. PMID: 30217009 Free PMC article.
-
Structures and Ribosomal Interaction of Ribosome-Inactivating Proteins.Molecules. 2016 Nov 21;21(11):1588. doi: 10.3390/molecules21111588. Molecules. 2016. PMID: 27879643 Free PMC article. Review.
-
Structures of eukaryotic ribosomal stalk proteins and its complex with trichosanthin, and their implications in recruiting ribosome-inactivating proteins to the ribosomes.Toxins (Basel). 2015 Feb 25;7(3):638-47. doi: 10.3390/toxins7030638. Toxins (Basel). 2015. PMID: 25723321 Free PMC article. Review.
Cited by
-
Ricin uses arginine 235 as an anchor residue to bind to P-proteins of the ribosomal stalk.Sci Rep. 2017 Feb 23;7:42912. doi: 10.1038/srep42912. Sci Rep. 2017. PMID: 28230053 Free PMC article.
-
Structural Analysis of Single Domain Antibodies Bound to a Second Neutralizing Hot Spot on Ricin Toxin's Enzymatic Subunit.J Biol Chem. 2017 Jan 20;292(3):872-883. doi: 10.1074/jbc.M116.758102. Epub 2016 Nov 30. J Biol Chem. 2017. PMID: 27903650 Free PMC article.
-
Localization of non-linear neutralizing B cell epitopes on ricin toxin's enzymatic subunit (RTA).Immunol Lett. 2014 Mar-Apr;158(1-2):7-13. doi: 10.1016/j.imlet.2013.11.009. Epub 2013 Nov 21. Immunol Lett. 2014. PMID: 24269767 Free PMC article.
-
The ribosome-inactivating proteins MAP30 and Momordin inhibit SARS-CoV-2.PLoS One. 2023 Jun 29;18(6):e0286370. doi: 10.1371/journal.pone.0286370. eCollection 2023. PLoS One. 2023. PMID: 37384752 Free PMC article.
-
Recent advances in the development of vaccines against ricin.Hum Vaccin Immunother. 2016 May 3;12(5):1196-201. doi: 10.1080/21645515.2015.1124202. Epub 2016 Jan 25. Hum Vaccin Immunother. 2016. PMID: 26810367 Free PMC article.
References
-
- Audi J., Belson M., Patel M., Schier J., Osterloh J. (2005) Ricin poisoning. A comprehensive review. JAMA 294, 2342–2351 - PubMed
-
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28 S ribosomal RNA caused by the toxins. J. Biol. Chem. 262, 5908–5912 - PubMed
-
- Olsnes S., Refsnes K., Pihl A. (1974) Mechanism of action of the toxic lectins abrin and ricin. Nature 249, 627–631 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

