Evidence for a diffusible factor that induces susceptibility in the Colletotrichum-maize disease interaction
- PMID: 24003973
- PMCID: PMC6638722
- DOI: 10.1111/mpp.12069
Evidence for a diffusible factor that induces susceptibility in the Colletotrichum-maize disease interaction
Abstract
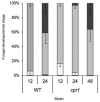
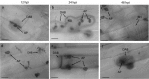
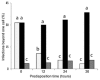
Colletotrichum graminicola, the causal agent of maize anthracnose, is a hemibiotrophic fungus that initially infects living host cells via primary hyphae surrounded by a membrane. A nonpathogenic mutant disrupted in a gene encoding a component of the signal peptidase complex, and believed to be deficient in protein processing and secretion, regained pathogenicity when it was inoculated onto maize leaf sheaths close to the wild-type fungus. Evidence is presented suggesting that the wild-type produces a diffusible factor(s) that induces the localized susceptibility of host cells at the borders of expanding colonies, causing them to become receptive to biotrophic invasion. The induced susceptibility effect is limited to a distance of approximately eight cells from the edge of the wild-type colony, is dosage dependent and is specific to C. graminicola.
© 2013 BSPP AND JOHN WILEY & SONS LTD.
Figures


Similar articles
-
A highly conserved metalloprotease effector enhances virulence in the maize anthracnose fungus Colletotrichum graminicola.Mol Plant Pathol. 2016 Sep;17(7):1048-62. doi: 10.1111/mpp.12347. Epub 2016 Apr 4. Mol Plant Pathol. 2016. PMID: 26619206 Free PMC article.
-
Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotricum graminicola in maize.Plant Physiol. 2012 Mar;158(3):1342-58. doi: 10.1104/pp.111.190397. Epub 2012 Jan 12. Plant Physiol. 2012. PMID: 22247271 Free PMC article.
-
Infection structure-specific reductive iron assimilation is required for cell wall integrity and full virulence of the maize pathogen Colletotrichum graminicola.Mol Plant Microbe Interact. 2013 Jun;26(6):695-708. doi: 10.1094/MPMI-01-13-0003-R. Mol Plant Microbe Interact. 2013. PMID: 23639025
-
Maize Anthracnose Stalk Rot in the Genomic Era.Plant Dis. 2022 Sep;106(9):2281-2298. doi: 10.1094/PDIS-10-21-2147-FE. Epub 2022 Aug 12. Plant Dis. 2022. PMID: 35291814 Review.
-
Fungal Pathogenesis-Related Cell Wall Biogenesis, with Emphasis on the Maize Anthracnose Fungus Colletotrichum graminicola.Plants (Basel). 2022 Mar 23;11(7):849. doi: 10.3390/plants11070849. Plants (Basel). 2022. PMID: 35406829 Free PMC article. Review.
Cited by
-
A comparative genomic analysis of putative pathogenicity genes in the host-specific sibling species Colletotrichum graminicola and Colletotrichum sublineola.BMC Genomics. 2017 Jan 10;18(1):67. doi: 10.1186/s12864-016-3457-9. BMC Genomics. 2017. PMID: 28073340 Free PMC article.
-
Identification of effector CEP112 that promotes the infection of necrotrophic Alternaria solani.BMC Plant Biol. 2022 Sep 29;22(1):466. doi: 10.1186/s12870-022-03845-w. BMC Plant Biol. 2022. PMID: 36171557 Free PMC article.
-
Identification and functional analysis of protein secreted by Alternaria solani.PLoS One. 2023 Mar 6;18(3):e0281530. doi: 10.1371/journal.pone.0281530. eCollection 2023. PLoS One. 2023. PMID: 36877688 Free PMC article.
-
A Colletotrichum graminicola mutant deficient in the establishment of biotrophy reveals early transcriptional events in the maize anthracnose disease interaction.BMC Genomics. 2016 Mar 8;17:202. doi: 10.1186/s12864-016-2546-0. BMC Genomics. 2016. PMID: 26956617 Free PMC article.
References
-
- Amselem, J. , Cuomo, C.A. , van Kan, J.A.L. , Viaud, M. , Benito, E.P. , Couloux, A. , Coutinho, P.M. , de Vriesm, R.P. , Dyer, P.S. , Fillinger, S. , Fournier, E. , Gout, L. , Hahn, M. , Kohn, L. , Lapalu, N. , Plummer, K.M. , Pradier, J.M. , Quévillon, E. , Sharon, A. , Simon, A. , ten Have, A. , Tudzynski, B. , Tudzynski, P. , Wincker, P. , Andrew, M. , Anthouard, V. , Beever, R.E. , Beffa, R. , Benoit, I. , Bouzid, O. , Brault, B. , Chen, Z. , Choquer, M. , Collémare, J. , Cotton, P. , Danchin, E.G. , Da Silva, C. , Gautier, A. , Giraud, C. , Giraud, T. , Gonzalez, C. , Grossetete, S. , Güldener, U. , Henrissat, B. , Howlett, B.J. , Kodira, C. , Kretschmer, M. , Lappartient, A. , Leroch, M. , Levis, C. , Mauceli, E. , Neuvéglise, C. , Oeser, B. , Pearson, M. , Poulain, J. , Poussereau, N. , Quesneville, H. , Rascle, C. , Schumacher, J. , Ségurens, B. , Sexton, A. , Silva, E. , Sirven, C. , Soanes, D.M. , Talbot, N.J. , Templeton, M. , Yandava, C. , Yarden, O. , Zeng, Q. , Rollins, J.A. , Lebrun, M.H. and Dickman, M. (2011) Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea . PLoS Genet. 7, e1002230. - PMC - PubMed
-
- Andrie, R.M. , Martinez, J.P. and Ciuffetti, L.M. (2005) Development of ToxA and ToxB promoter‐driven fluorescent protein expression vectors for use in filamentous ascomycetes. Mycologia, 97, 1152–1161. - PubMed
-
- Bell, A. and Presley, J. (1969) Heat‐inhibited or heat‐killed conidia of Verticillium albo‐atrum induce disease resistance and phytoalexin synthesis in cotton. Phytopathology, 59, 1147–1151.
-
- Benito, E.P. , ten Have, A. , van't Klooster, J.W. and van Kan, J.A. (1998) Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea . Eur. J. Plant Pathol. 104, 207–220.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

