The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury
- PMID: 24004276
- PMCID: PMC3904530
- DOI: 10.1089/neu.2013.3108
The cancer drug tamoxifen: a potential therapeutic treatment for spinal cord injury
Abstract
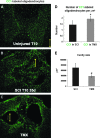
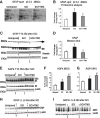
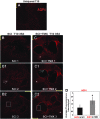
Tamoxifen (TMX) is a selective estrogen receptor modulator that can mimic the neuroprotective effects of estrogen but lacks its systemic adverse effects. We found that TMX (1 mg/day) significantly improved the motor recovery of partially paralyzed hind limbs of male adult rats with thoracic spinal cord injury (SCI), thus indicating a translational potential for this cancer medication given its clinical safety and applicability and the lack of currently available treatments for SCI. To shed light on the mechanisms underlying the beneficial effects of TMX for SCI, we used proteomic analyses, Western blots and histological assays, which showed that TMX treatment spared mature oligodendrocytes/increased myelin levels and altered reactive astrocytes, including the upregulation of the water channels aquaporin 4 (AQP4), a novel finding. AQP4 increases in TMX-treated SCI rats were associated with smaller fluid-filled cavities with borders consisting of densely packed AQP4-expressing astrocytes that closely resemble the organization of normal glia limitans externa (in contrast to large cavities in control SCI rats that lacked glia limitans-like borders and contained reactive glial cells). Based on our findings, we propose that TMX is a promising candidate for the therapeutic treatment of SCI and a possible intervention for other neuropathological conditions associated with demyelination and AQP4 dysfunction.
Figures





References
-
- Amtul Z., Wang L., Westaway D., and Rozmahel R. F. (2010). Neuroprotective mechanism conferred by 17beta-estradiol on the biochemical basis of Alzheimer's disease. Neuroscience 169, 781–786 - PubMed
-
- Bourque M., Dluzen D. E., and Di Paolo T. (2009). Neuroprotective actions of sex steroids in Parkinson's disease. Front Neuroendocrinol. 30, 142–157 - PubMed
-
- Garcia-Segura L. M. and Melcangi R. C. (2006). Steroids and glial cell function. Glia 54, 485–498 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

