Comparison of once-daily versus twice-daily combination of ropinirole prolonged release in Parkinson's disease
- PMID: 24004540
- PMCID: PMC3766261
- DOI: 10.1186/1471-2377-13-113
Comparison of once-daily versus twice-daily combination of ropinirole prolonged release in Parkinson's disease
Abstract
Background: Ropinirole prolonged release (RPR) is a once-daily formulation. However, there may be individual pharmacokinetic differences so that multiple dosing may be preferred in some individuals. This study compares once-daily and twice-daily RPR in patients with Parkinson's disease.
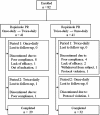
Methods: This study was an open-label crossover study. We enrolled Parkinson's disease patients on dopamine agonist therapy with unsatisfactory control such as motor fluctuation, dyskinesia and sleep-related problems. Agonists were switched into equivalent dose of RPR. Subjects were consecutively enrolled into either once-daily first or twice-daily first groups, and received the same amount of RPR in a single and two divided dosing for 8 weeks respectively in a crossover manner without a washout period.The primary outcome was a questionnaire of the preference completed by patients in the last visit. The secondary outcome measures included the Unified Parkinson's Disease Rating Scale part 3 (mUPDRS), Hoehn and Yahr stage (H&Y); sleep questionnaire including overall quality of sleep, nocturnal off symptoms and early morning symptoms; Epworth Sleep Scale (ESS); compliances and patient global impression (PGI).
Results: A total of 82 patients were enrolled and 61 completed the study. 31 patients preferred twice-daily regimen, 17 preferred the once-daily regimen, and 13 had no preference. Their mean mUPDRS, H&Y, ESS, sleep quality, compliance and adverse events were not statistically different in both regimens. PGI-improvement on wearing off defined was better in twice-daily dosing regimen.
Conclusions: RPR is a once-daily formulation, but multiple dosing was preferred in many patients. Multiple dosing of RPR might be a therapeutic option if once-daily dosing is unsatisfactory.
Trial registration: ClinicalTrials.gov NCT00986245.
Figures
References
-
- Obeso JA, Rodriguez-Oroz MC, Chana P, Lera G, Rodriguez M, Olanow CW. The evolution and origin of motor complications in Parkinson’s disease. Neurology. 2000;55(11 Suppl 4):S13–S20. discussion S21-13. - PubMed
-
- Stocchi F, Hersh BP, Scott BL, Nausieda PA, Giorgi L, Ease PDMSI. Ropinirole 24-hour prolonged release and ropinirole immediate release in early Parkinson’s disease: a randomized, double-blind, non-inferiority crossover study. Curr Med Res Opin. 2008;24(10):2883–2895. doi: 10.1185/03007990802387130. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical