Lipid tubule growth by osmotic pressure
- PMID: 24004559
- PMCID: PMC3785832
- DOI: 10.1098/rsif.2013.0637
Lipid tubule growth by osmotic pressure
Abstract
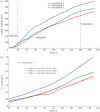
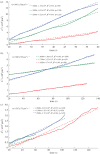
We present here a procedure for growing lipid tubules in vitro. This method allows us to grow tubules of consistent shape and structure, and thus can be a useful tool for nano-engineering applications. There are three stages during the tubule growth process: initiation, elongation and termination. Balancing the forces that act on the tubule head shows that the growth of tubules during the elongation phase depends on the balance between osmotic pressure and the viscous drag exerted on the membrane from the substrate and the external fluid. Using a combination of mathematical modelling and experiment, we identify the key forces that control tubule growth during the elongation phase.
Keywords: lipid membranes; lipid tubules; vesicles.
Figures



Similar articles
-
Engineering lipid tubules using nano-sized building blocks: the combinatorial self-assembly of vesicles.Lab Chip. 2008 Feb;8(2):339-45. doi: 10.1039/b713930f. Epub 2007 Dec 12. Lab Chip. 2008. PMID: 18231675
-
Nonlocal strain gradient beam model for postbuckling and associated vibrational response of lipid supramolecular protein micro/nano-tubules.Math Biosci. 2018 Jan;295:24-35. doi: 10.1016/j.mbs.2017.11.002. Epub 2017 Nov 8. Math Biosci. 2018. PMID: 29104135
-
Chemically triggered ejection of membrane tubules controlled by intermonolayer friction.Phys Rev Lett. 2009 Jan 9;102(1):018102. doi: 10.1103/PhysRevLett.102.018102. Epub 2009 Jan 7. Phys Rev Lett. 2009. PMID: 19257244
-
Measurement of lipid forces by X-ray diffraction and osmotic stress.Methods Mol Biol. 2007;400:405-19. doi: 10.1007/978-1-59745-519-0_27. Methods Mol Biol. 2007. PMID: 17951749 Review.
-
Colloidal aspects of digestion of Pickering emulsions: Experiments and theoretical models of lipid digestion kinetics.Adv Colloid Interface Sci. 2019 Jan;263:195-211. doi: 10.1016/j.cis.2018.10.002. Epub 2018 Oct 22. Adv Colloid Interface Sci. 2019. PMID: 30580767 Review.
Cited by
-
Physical mechanisms of platelet formation.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):21841-21843. doi: 10.1073/pnas.2014390117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788351 Free PMC article. No abstract available.
-
Imaging non-classical mechanical responses of lipid membranes using molecular rotors.Chem Sci. 2020 Dec 22;12(7):2604-2613. doi: 10.1039/d0sc05874b. Chem Sci. 2020. PMID: 34164028 Free PMC article.
-
The nematocyst's sting is driven by the tubule moving front.J R Soc Interface. 2017 Mar;14(128):20160917. doi: 10.1098/rsif.2016.0917. J R Soc Interface. 2017. PMID: 28250103 Free PMC article.
-
The Mechanics and Thermodynamics of Tubule Formation in Biological Membranes.J Membr Biol. 2021 Jun;254(3):273-291. doi: 10.1007/s00232-020-00164-9. Epub 2021 Jan 19. J Membr Biol. 2021. PMID: 33462667 Free PMC article. Review.
References
-
- Zhang XJ, Fang JY. 2010. Assembly of vesicles into fractal and prong patterns on substrates. Soft Matter 6, 2139–2142. (10.1039/c001210f) - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

