The control of branching morphogenesis
- PMID: 24004663
- PMCID: PMC3787747
- DOI: 10.1098/rsob.130088
The control of branching morphogenesis
Abstract
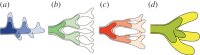
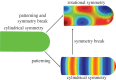
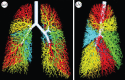
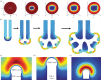
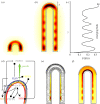
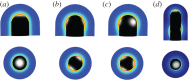
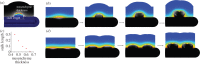
Many organs of higher organisms are heavily branched structures and arise by an apparently similar process of branching morphogenesis. Yet the regulatory components and local interactions that have been identified differ greatly in these organs. It is an open question whether the regulatory processes work according to a common principle and how far physical and geometrical constraints determine the branching process. Here, we review the known regulatory factors and physical constraints in lung, kidney, pancreas, prostate, mammary gland and salivary gland branching morphogenesis, and describe the models that have been formulated to analyse their impacts.
Keywords: branching; computational modelling; in silico organogenesis.
Figures







References
-
- Affolter M, Zeller R, Caussinus E. 2009. Tissue remodelling through branching morphogenesis. Nat. Rev. Mol. Cell Biol. 10, 831–842 - PubMed
-
- Metzger RJ, Klein OD, Martin GR, Krasnow MA. 2008. The branching programme of mouse lung development. Nature 453, 745–750 (doi:10.1038/nature07005) - DOI - PMC - PubMed
-
- Blanc P, Coste K, Pouchin P, Azas J-M, Blanchon L, Gallot D, Sapin V. 2012. A role for mesenchyme dynamics in mouse lung branching morphogenesis. PLoS ONE 7, e41643 (doi:10.1371/journal.pone.0041643) - DOI - PMC - PubMed
-
- Watanabe T, Costantini F. 2004. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev. Biol. 271, 98–108 (doi:10.1016/j.ydbio.2004.03.025) - DOI - PubMed
-
- Meyer TN, Schwesinger C, Bush KT, Stuart RO, Rose DW, Shah MM, Vaughn DA, Steer DL, Nigam SK. 2004. Spatiotemporal regulation of morphogenetic molecules during in vitro branching of the isolated ureteric bud: toward a model of branching through budding in the developing kidney. Dev. Biol. 275, 44–67 (doi:10.1016/j.ydbio.2004.07.022) - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

