Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac
- PMID: 24004946
- PMCID: PMC3775417
- DOI: 10.1242/dev.096255
Dynamic responses of endothelial cells to changes in blood flow during vascular remodeling of the mouse yolk sac
Abstract
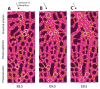
Despite extensive work showing the importance of blood flow in angiogenesis and vessel remodeling, very little is known about how changes in vessel diameter are orchestrated at the cellular level in response to mechanical forces. To define the cellular changes necessary for remodeling, we performed live confocal imaging of cultured mouse embryos during vessel remodeling. Our data revealed that vessel diameter increase occurs via two distinct processes that are dependent on normal blood flow: vessel fusions and directed endothelial cell migrations. Vessel fusions resulted in a rapid change in vessel diameter and were restricted to regions that experience the highest flow near the vitelline artery and vein. Directed cell migrations induced by blood flow resulted in the recruitment of endothelial cells to larger vessels from smaller capillaries and were observed in larger artery segments as they expanded. The dynamic and specific endothelial cell behaviors captured in this study reveal how sensitive endothelial cells are to changes in blood flow and how such responses drive vascular remodeling.
Keywords: Cell migration; Endothelial cell; Mouse; Vascular remodeling; Vessel fusion; Yolk sac.
Figures

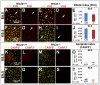
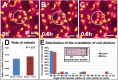
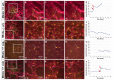

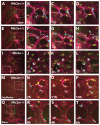

References
-
- Argraves W. S., Drake C. J. (2005). Genes critical to vasculogenesis as defined by systematic analysis of vascular defects in knockout mice. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 286, 875–884 - PubMed
-
- Califano J. P., Reinhart-King C. A. (2010). Exogenous and endogenous force regulation of endothelial cell behavior. J. Biomech. 43, 79–86 - PubMed
-
- Chatzizisis Y. S., Coskun A. U., Jonas M., Edelman E. R., Feldman C. L., Stone P. H. (2007). Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49, 2379–2393 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

