Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo
- PMID: 24005123
- PMCID: PMC4204630
- DOI: 10.1001/jamapsychiatry.2013.1969
Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders: a randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo
Abstract
Importance: Strategies to improve the course of recurrent major depressive disorder have great public health relevance. To reduce the risk of relapse/recurrence after acute phase cognitive therapy (CT), a continuation phase model of therapy may improve outcomes.
Objectives: To test the efficacy of continuation phase CT (C-CT) and fluoxetine for relapse prevention in a pill placebo (PBO)-controlled randomized trial and compare the durability of prophylaxis after discontinuation of treatments.
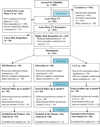
Design: A sequential, 3-stage design with an acute phase (all patients received 12 weeks of CT); 8-month experimental phase (responders at higher risk were randomized to C-CT, fluoxetine, or PBO); and 24 months of longitudinal, posttreatment follow-up.
Setting: Two university-based specialty clinics.
Patients: A total of 523 adults with recurrent major depressive disorder began acute phase CT, of which 241 higher-risk responders were randomized and 181 subsequently entered the follow-up.
Interventions: Cognitive therapy responders at higher risk for relapse were randomized to receive 8 months of C-CT (n = 86), fluoxetine (n = 86), or PBO (n = 69).
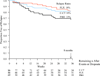
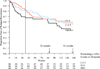
Main outcomes and measures: Survival analyses of relapse/recurrence rates, as determined by blinded evaluators using DSM-IV criteria and the Longitudinal Interval Follow-up Evaluation. RESULTS As predicted, the C-CT or fluoxetine groups were significantly less likely to relapse than the PBO group across 8 months. Relapse/recurrence rates for C-CT and fluoxetine were nearly identical during the 8 months of treatment, although C-CT patients were more likely to accept randomization, stayed in treatment longer, and attended more sessions than those in the fluoxetine and PBO groups. Contrary to prediction, relapse/recurrence rates following the discontinuation of C-CT and fluoxetine did not differ.
Conclusions and relevance: Relapse risk was reduced by both C-CT and fluoxetine in an enriched randomization sampling only CT responders. The preventive effects of C-CT were not significantly more durable than those of fluoxetine after treatment was stopped, suggesting that some higher-risk patients may require alternate longer-term interventions.
Trial registration: clinicaltrials.gov Identifiers: NCT00118404, NCT00183664, and NCT00218764.
Figures
- -
FLX vs. PBO (χ12 = 3.92, p-value = .02)
- -
C-CT vs. PBO (χ12 = 3.39, p-value = .03)
- -
C-CT vs. FLX (χ12 = 0.04, p-value = .42)
Comment in
-
Continuation-phase cognitive therapy and fluoxetine are effective in reducing the risk of relapse/recurrence in major depression after incomplete remission.Evid Based Ment Health. 2014 May;17(2):59-60. doi: 10.1136/eb-2013-101715. Epub 2014 Mar 31. Evid Based Ment Health. 2014. PMID: 24688091 No abstract available.
References
-
- Coryell W, Scheftner W, Keller M, Endicott J, et al. The enduring psychosocial consequences of mania and depression. Am. J. Psychiatry. 1993 May;150(5):720–727. - PubMed
-
- Judd LL, Akiskal HS, Maser JD, et al. Major depressive disorder: A prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J. Affect. Disord. 1998 Sep;50(2–3):97–108. - PubMed
-
- Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA. 2003 Jun 18;289(23):3152–3160. - PubMed
-
- American Psychiatric Association. Practice Guideline for the treatment of patients with Major Depressive Disorder in Adults. American Psychiatric Association; 1993. p. 51.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

