Tau pathology as a cause and consequence of the UPR
- PMID: 24005281
- PMCID: PMC6618381
- DOI: 10.1523/JNEUROSCI.2961-13.2013
Tau pathology as a cause and consequence of the UPR
Figures
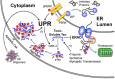
Comment on
-
Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation.J Neurosci. 2013 May 29;33(22):9498-507. doi: 10.1523/JNEUROSCI.5397-12.2013. J Neurosci. 2013. PMID: 23719816 Free PMC article.
References
-
- Abisambra JF, Jinwal UK, Blair LJ, O'Leary JC, 3rd, Li Q, Brady S, Wang L, Guidi CE, Zhang B, Nordhues BA, Cockman M, Suntharalingham A, Li P, Jin Y, Atkins CA, Dickey CA. Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci. 2013;33:9498–9507. doi: 10.1523/JNEUROSCI.5397-12.2013. - DOI - PMC - PubMed
-
- Hawkins BE, Krishnamurthy S, Castillo-Carranza DL, Sengupta U, Prough DS, Jackson GR, DeWitt DS, Kayed R. Rapid accumulation of endogenous tau oligomers in a rat model of traumatic brain injury: possible link between traumatic brain injury and sporadic tauopathies. J Biol Chem. 2013;288:17042–17050. doi: 10.1074/jbc.M113.472746. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
