A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons
- PMID: 24005292
- PMCID: PMC3761049
- DOI: 10.1523/JNEUROSCI.2045-13.2013
A reorganized GABAergic circuit in a model of epilepsy: evidence from optogenetic labeling and stimulation of somatostatin interneurons
Abstract
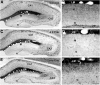
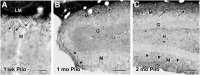
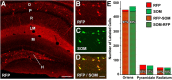
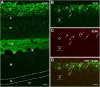
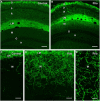
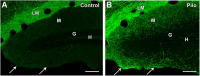
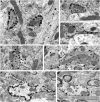
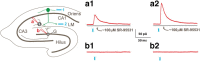
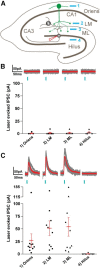
Axonal sprouting of excitatory neurons is frequently observed in temporal lobe epilepsy, but the extent to which inhibitory interneurons undergo similar axonal reorganization remains unclear. The goal of this study was to determine whether somatostatin (SOM)-expressing neurons in stratum (s.) oriens of the hippocampus exhibit axonal sprouting beyond their normal territory and innervate granule cells of the dentate gyrus in a pilocarpine model of epilepsy. To obtain selective labeling of SOM-expressing neurons in s. oriens, a Cre recombinase-dependent construct for channelrhodopsin2 fused to enhanced yellow fluorescent protein (ChR2-eYFP) was virally delivered to this region in SOM-Cre mice. In control mice, labeled axons were restricted primarily to s. lacunosum-moleculare. However, in pilocarpine-treated animals, a rich plexus of ChR2-eYFP-labeled fibers and boutons extended into the dentate molecular layer. Electron microscopy with immunogold labeling demonstrated labeled axon terminals that formed symmetric synapses on dendritic profiles in this region, consistent with innervation of granule cells. Patterned illumination of ChR2-labeled fibers in s. lacunosum-moleculare of CA1 and the dentate molecular layer elicited GABAergic inhibitory responses in dentate granule cells in pilocarpine-treated mice but not in controls. Similar optical stimulation in the dentate hilus evoked no significant responses in granule cells of either group of mice. These findings indicate that under pathological conditions, SOM/GABAergic neurons can undergo substantial axonal reorganization beyond their normal territory and establish aberrant synaptic connections. Such reorganized circuitry could contribute to functional deficits in inhibition in epilepsy, despite the presence of numerous GABAergic terminals in the region.
Figures
Similar articles
-
Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy.Epilepsia. 2011 Nov;52(11):2057-64. doi: 10.1111/j.1528-1167.2011.03253.x. Epub 2011 Aug 29. Epilepsia. 2011. PMID: 21883182 Free PMC article.
-
Hilar somatostatin interneuron loss reduces dentate gyrus inhibition in a mouse model of temporal lobe epilepsy.Epilepsia. 2016 Jun;57(6):977-83. doi: 10.1111/epi.13376. Epub 2016 Mar 31. Epilepsia. 2016. PMID: 27030321 Free PMC article.
-
Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy.J Neurosci. 2009 Nov 11;29(45):14247-56. doi: 10.1523/JNEUROSCI.3842-09.2009. J Neurosci. 2009. PMID: 19906972 Free PMC article.
-
Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy.Epilepsia. 2012 Jun;53 Suppl 1:98-108. doi: 10.1111/j.1528-1167.2012.03481.x. Epilepsia. 2012. PMID: 22612814 Review.
-
Epileptogenesis in the dentate gyrus: a critical perspective.Prog Brain Res. 2007;163:755-73. doi: 10.1016/S0079-6123(07)63041-6. Prog Brain Res. 2007. PMID: 17765749 Review.
Cited by
-
Cortical interneuron development is affected in 4H leukodystrophy.Brain. 2023 Jul 3;146(7):2846-2860. doi: 10.1093/brain/awad017. Brain. 2023. PMID: 36729681 Free PMC article.
-
Brain-wide reconstruction of inhibitory circuits after traumatic brain injury.Nat Commun. 2022 Jun 14;13(1):3417. doi: 10.1038/s41467-022-31072-2. Nat Commun. 2022. PMID: 35701434 Free PMC article.
-
Reclusive chandeliers: Functional isolation of dentate axo-axonic cells after experimental status epilepticus.Prog Neurobiol. 2023 Dec;231:102542. doi: 10.1016/j.pneurobio.2023.102542. Epub 2023 Oct 26. Prog Neurobiol. 2023. PMID: 37898313 Free PMC article.
-
Specificity, Versatility, and Continual Development: The Power of Optogenetics for Epilepsy Research.Front Cell Neurosci. 2018 Jun 14;12:151. doi: 10.3389/fncel.2018.00151. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29962936 Free PMC article. Review.
-
Comparative density of CCK- and PV-GABA cells within the cortex and hippocampus.Front Neuroanat. 2015 Sep 23;9:124. doi: 10.3389/fnana.2015.00124. eCollection 2015. Front Neuroanat. 2015. PMID: 26441554 Free PMC article.
References
-
- Boulland JL, Ferhat L, Tallak Solbu T, Ferrand N, Chaudhry FA, Storm-Mathisen J, Esclapez M. Changes in vesicular transporters for gamma-aminobutyric acid and glutamate reveal vulnerability and reorganization of hippocampal neurons following pilocarpine-induced seizures. J Comp Neurol. 2007;503:466–485. doi: 10.1002/cne.21384. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
