Synaptotagmin interaction with SNAP-25 governs vesicle docking, priming, and fusion triggering
- PMID: 24005294
- PMCID: PMC4104293
- DOI: 10.1523/JNEUROSCI.1236-13.2013
Synaptotagmin interaction with SNAP-25 governs vesicle docking, priming, and fusion triggering
Abstract
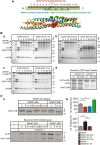
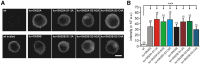
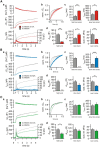
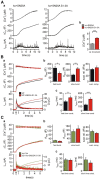
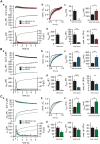
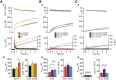
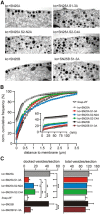
SNARE complex assembly constitutes a key step in exocytosis that is rendered Ca(2+)-dependent by interactions with synaptotagmin-1. Two putative sites for synaptotagmin binding have recently been identified in SNAP-25 using biochemical methods: one located around the center and another at the C-terminal end of the SNARE bundle. However, it is still unclear whether and how synaptotagmin-1 × SNARE interactions at these sites are involved in regulating fast neurotransmitter release. Here, we have used electrophysiological techniques with high time-resolution to directly investigate the mechanistic ramifications of proposed SNAP-25 × synaptotagmin-1 interaction in mouse chromaffin cells. We demonstrate that the postulated central binding domain surrounding layer zero covers both SNARE motifs of SNAP-25 and is essential for vesicle docking, priming, and fast fusion-triggering. Mutation of this site caused no further functional alterations in synaptotagmin-1-deficient cells, indicating that the central acidic patch indeed constitutes a mechanistically relevant synaptotagmin-1 interaction site. Moreover, our data show that the C-terminal binding interface only plays a subsidiary role in triggering but is required for the full size of the readily releasable pool. Intriguingly, we also found that mutation of synaptotagmin-1 interaction sites led to more pronounced phenotypes in the context of the adult neuronal isoform SNAP-25B than in the embryonic isoform SNAP-25A. Further experiments demonstrated that stronger synaptotagmin-1 × SNAP-25B interactions allow for the larger primed vesicle pool supported by SNAP-25 isoform B. Thus, synaptotagmin-1 × SNARE interactions are not only required for multiple mechanistic steps en route to fusion but also underlie the developmental control of the releasable vesicle pool.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
