Neural representation of a target auditory memory in a cortico-basal ganglia pathway
- PMID: 24005299
- PMCID: PMC3761053
- DOI: 10.1523/JNEUROSCI.0710-13.2013
Neural representation of a target auditory memory in a cortico-basal ganglia pathway
Abstract
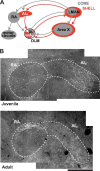
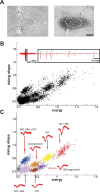
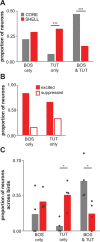
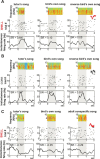
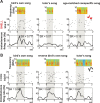
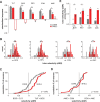
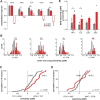
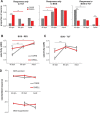
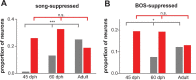
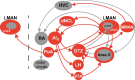
Vocal learning in songbirds, like speech acquisition in humans, entails a period of sensorimotor integration during which vocalizations are evaluated via auditory feedback and progressively refined to achieve an imitation of memorized vocal sounds. This process requires the brain to compare feedback of current vocal behavior to a memory of target vocal sounds. We report the discovery of two distinct populations of neurons in a cortico-basal ganglia circuit of juvenile songbirds (zebra finches, Taeniopygia guttata) during vocal learning: (1) one in which neurons are selectively tuned to memorized sounds and (2) another in which neurons are selectively tuned to self-produced vocalizations. These results suggest that neurons tuned to learned vocal sounds encode a memory of those target sounds, whereas neurons tuned to self-produced vocalizations encode a representation of current vocal sounds. The presence of neurons tuned to memorized sounds is limited to early stages of sensorimotor integration: after learning, the incidence of neurons encoding memorized vocal sounds was greatly diminished. In contrast to this circuit, neurons known to drive vocal behavior through a parallel cortico-basal ganglia pathway show little selective tuning until late in learning. One interpretation of these data is that representations of current and target vocal sounds in the shell circuit are used to compare ongoing patterns of vocal feedback to memorized sounds, whereas the parallel core circuit has a motor-related role in learning. Such a functional subdivision is similar to mammalian cortico-basal ganglia pathways in which associative-limbic circuits mediate goal-directed responses, whereas sensorimotor circuits support motor aspects of learning.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
