Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat
- PMID: 24005302
- PMCID: PMC3761054
- DOI: 10.1523/JNEUROSCI.1980-13.2013
Central activation of the A1 adenosine receptor (A1AR) induces a hypothermic, torpor-like state in the rat
Abstract
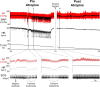
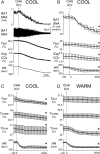
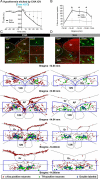
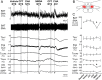
Since central activation of A1 adenosine receptors (A1ARs) plays an important role in the induction of the hypothermic and hypometabolic torpid state in hibernating mammals, we investigated the potential for the A1AR agonist N6-cyclohexyladenosine to induce a hypothermic, torpor-like state in the (nonhibernating) rat. Core and brown adipose tissue temperatures, EEG, heart rate, and arterial pressure were recorded in free-behaving rats, and c-fos expression in the brain was analyzed, following central administration of N6-cyclohexyladenosine. Additionally, we recorded the sympathetic nerve activity to brown adipose tissue; expiratory CO2 and skin, core, and brown adipose tissue temperatures; and shivering EMGs in anesthetized rats following central and localized, nucleus of the solitary tract, administration of N6-cyclohexyladenosine. In rats exposed to a cool (15°C) ambient temperature, central A1AR stimulation produced a torpor-like state similar to that in hibernating species and characterized by a marked fall in body temperature due to an inhibition of brown adipose tissue and shivering thermogenesis that is mediated by neurons in the nucleus of the solitary tract. During the induced hypothermia, EEG amplitude and heart rate were markedly reduced. Skipped heartbeats and transient bradycardias occurring during the hypothermia were vagally mediated since they were eliminated by systemic muscarinic receptor blockade. These findings demonstrate that a deeply hypothermic, torpor-like state can be pharmacologically induced in a nonhibernating mammal and that recovery of normothermic homeostasis ensues upon rewarming. These results support the potential for central activation of A1ARs to be used in the induction of a hypothermic, therapeutically beneficial state in humans.
Figures




Similar articles
-
Translating drug-induced hibernation to therapeutic hypothermia.ACS Chem Neurosci. 2015 Jun 17;6(6):899-904. doi: 10.1021/acschemneuro.5b00056. Epub 2015 Apr 8. ACS Chem Neurosci. 2015. PMID: 25812681 Free PMC article.
-
Thermoregulatory inversion: a novel thermoregulatory paradigm.Am J Physiol Regul Integr Comp Physiol. 2017 May 1;312(5):R779-R786. doi: 10.1152/ajpregu.00022.2017. Epub 2017 Mar 22. Am J Physiol Regul Integr Comp Physiol. 2017. PMID: 28330964 Free PMC article.
-
Central activation of the A1 adenosine receptor in fed mice recapitulates only some of the attributes of daily torpor.J Comp Physiol B. 2017 Jul;187(5-6):835-845. doi: 10.1007/s00360-017-1084-7. Epub 2017 Apr 4. J Comp Physiol B. 2017. PMID: 28378088 Free PMC article.
-
Hibernation, Hypothermia and a Possible Therapeutic "Shifted Homeostasis" Induced by Central Activation of A1 Adenosine Receptor (A1AR).Nihon Shinkei Seishin Yakurigaku Zasshi. 2016 Apr;36(2):51-4. Nihon Shinkei Seishin Yakurigaku Zasshi. 2016. PMID: 27333659 Free PMC article. Review.
-
[Pharmacological aspects of mammalian hibernation: central thermoregulation factors in hibernation cycle].Nihon Yakurigaku Zasshi. 2000 Nov;116(5):304-12. doi: 10.1254/fpj.116.304. Nihon Yakurigaku Zasshi. 2000. PMID: 11215381 Review. Japanese.
Cited by
-
Transcriptomic analysis of brown adipose tissue across the physiological extremes of natural hibernation.PLoS One. 2013 Dec 30;8(12):e85157. doi: 10.1371/journal.pone.0085157. eCollection 2013. PLoS One. 2013. PMID: 24386461 Free PMC article.
-
Identification of hypothermia-inducing neurons in the preoptic area and activation of them by isoflurane anesthesia and central injection of adenosine.J Physiol Sci. 2024 Jun 12;74(1):33. doi: 10.1186/s12576-024-00927-2. J Physiol Sci. 2024. PMID: 38867187 Free PMC article.
-
Induction of a torpor-like hypothermic and hypometabolic state in rodents by ultrasound.Nat Metab. 2023 May;5(5):789-803. doi: 10.1038/s42255-023-00804-z. Epub 2023 May 25. Nat Metab. 2023. PMID: 37231250 Free PMC article.
-
The relationship between fasting-induced torpor, sleep, and wakefulness in laboratory mice.Sleep. 2021 Sep 13;44(9):zsab093. doi: 10.1093/sleep/zsab093. Sleep. 2021. PMID: 33838033 Free PMC article.
-
Central neural regulation of brown adipose tissue thermogenesis and energy expenditure.Cell Metab. 2014 May 6;19(5):741-756. doi: 10.1016/j.cmet.2014.02.007. Epub 2014 Mar 13. Cell Metab. 2014. PMID: 24630813 Free PMC article. Review.
References
-
- Berner NJ, Heller HC. Does the preoptic anterior hypothalamus receive thermoafferent information? Am J Physiol. 1998;274:R9–R18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
