Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation
- PMID: 24005305
- PMCID: PMC6618384
- DOI: 10.1523/JNEUROSCI.1130-13.2013
Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation
Abstract
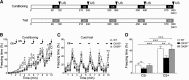
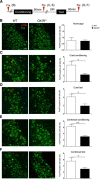
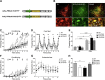
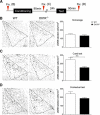
The noradrenergic (NA) projections arising from the locus ceruleus (LC) to the amygdala and bed nucleus of the stria terminalis have been implicated in the formation of emotional memory. Since NA neurons in the LC (LC-NA neurons) abundantly express orexin receptor-1 (OX1R) and receive prominent innervation by orexin-producing neurons, we hypothesized that an OX1R-mediated pathway is involved in the physiological fear learning process via regulation of LC-NA neurons. To evaluate this hypothesis, we examined the phenotype of Ox1r(-/-) mice in the classic cued and contextual fear-conditioning test. We found that Ox1r(-/-) mice showed impaired freezing responses in both cued and contextual fear-conditioning paradigms. In contrast, Ox2r(-/-) mice showed normal freezing behavior in the cued fear-conditioning test, while they exhibited shorter freezing time in the contextual fear-conditioning test. Double immunolabeling of Fos and tyrosine hydroxylase showed that double-positive LC-NA neurons after test sessions of both cued and contextual stimuli were significantly fewer in Ox1r(-/-) mice. AAV-mediated expression of OX1R in LC-NA neurons in Ox1r(-/-) mice restored the freezing behavior to the auditory cue to a comparable level to that in wild-type mice in the test session. Decreased freezing time during the contextual fear test was not affected by restoring OX1R expression in LC-NA neurons. These observations support the hypothesis that the orexin system modulates the formation and expression of fear memory via OX1R in multiple pathways. Especially, OX1R in LC-NA neurons plays an important role in cue-dependent fear memory formation and/or retrieval.
Figures

References
-
- Date Y, Ueta Y, Yamashita H, Yamaguchi H, Matsukura S, Kangawa K, Sakurai T, Yanagisawa M, Nakazato M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–753. doi: 10.1073/pnas.96.2.748. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
