Delta-opioid receptors mediate unique plasticity onto parvalbumin-expressing interneurons in area CA2 of the hippocampus
- PMID: 24005307
- PMCID: PMC6618379
- DOI: 10.1523/JNEUROSCI.0649-13.2013
Delta-opioid receptors mediate unique plasticity onto parvalbumin-expressing interneurons in area CA2 of the hippocampus
Abstract
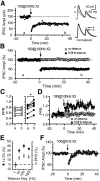
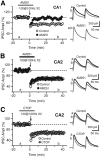
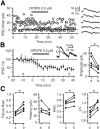
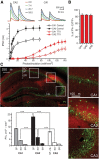
Inhibition is critical for controlling information transfer in the brain. However, the understanding of the plasticity and particular function of different interneuron subtypes is just emerging. Using acute hippocampal slices prepared from adult mice, we report that in area CA2 of the hippocampus, a powerful inhibitory transmission is acting as a gate to prevent CA3 inputs from driving CA2 neurons. Furthermore, this inhibition is highly plastic, and undergoes a long-term depression following high-frequency 10 Hz or theta-burst induction protocols. We describe a novel form of long-term depression at parvalbumin-expressing (PV+) interneuron synapses that is dependent on delta-opioid receptor (DOR) activation. Additionally, PV+ interneuron transmission is persistently depressed by DOR activation in area CA2 but only transiently depressed in area CA1. These results provide evidence for a differential temporal modulation of PV+ synapses between two adjacent cortical circuits, and highlight a new function of PV+ cells in controlling information transfer.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
