Self-propagation of pathogenic protein aggregates in neurodegenerative diseases
- PMID: 24005412
- PMCID: PMC3963807
- DOI: 10.1038/nature12481
Self-propagation of pathogenic protein aggregates in neurodegenerative diseases
Abstract
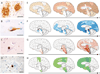
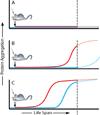
For several decades scientists have speculated that the key to understanding age-related neurodegenerative disorders may be found in the unusual biology of the prion diseases. Recently, owing largely to the advent of new disease models, this hypothesis has gained experimental momentum. In a remarkable variety of diseases, specific proteins have been found to misfold and aggregate into seeds that structurally corrupt like proteins, causing them to aggregate and form pathogenic assemblies ranging from small oligomers to large masses of amyloid. Proteinaceous seeds can therefore serve as self-propagating agents for the instigation and progression of disease. Alzheimer's disease and other cerebral proteopathies seem to arise from the de novo misfolding and sustained corruption of endogenous proteins, whereas prion diseases can also be infectious in origin. However, the outcome in all cases is the functional compromise of the nervous system, because the aggregated proteins gain a toxic function and/or lose their normal function. As a unifying pathogenic principle, the prion paradigm suggests broadly relevant therapeutic directions for a large class of currently intractable diseases.
Figures

References
-
- Malinovska L, Kroschwald S, Alberti S. Protein disorder, prion propensities, and self-organizing macromolecular collectives. Biochim. Biophys. Acta. 2013;1834:918–931. - PubMed
-
- Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. - PubMed
-
- Hartl FU, Bracher A, Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. - PubMed
-
- Thal DR, Rub U, Orantes M, Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

