Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats
- PMID: 24005529
- PMCID: PMC3947118
- DOI: 10.1007/s00213-013-3257-8
Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats
Abstract
Rationale: The "subjective high" from marijuana ingestion is likely due to Δ(9)-tetrahydrocannabinol (THC) activating the central cannabinoid receptor type 1 (CB1R) of the endocannabinoid signaling system. THC is a weak partial agonist according to in vitro assays, yet THC mimics the behavioral effects induced by more efficacious cannabinergics. This distinction may be important for understanding similarities and differences in the dose-effect spectra produced by marijuana/THC and designer cannabimimetics ("synthetic marijuana").
Objective: We evaluated if drug discrimination is able to functionally detect/differentiate between a full, high-efficacy CB1R agonist [(±)AM5983] and the low-efficacy agonist THC in vivo.
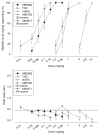
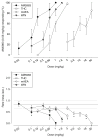
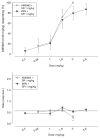
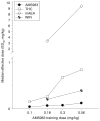
Materials and methods: Rats were trained to discriminate between four different doses of AM5983 (0.10 to 0.56 mg/kg), and vehicle and dose generalization curves were determined for both ligands at all four training doses of AM5983. The high-efficacy WIN55,212-2 and the lower-efficacy (R)-(+)-methanandamide were examined at some AM5983 training conditions. Antagonism tests involved rimonabant and WIN55,212-2 and AM5983. The separate (S)- and (R)-isomers of (±)AM5983 were tested at one AM5983 training dose (0.30 mg/kg). The in vitro cyclic adenosine monophosphate (cAMP) assay examined AM5983 and the known CB1R agonist CP55,940.
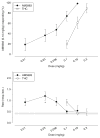
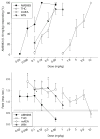
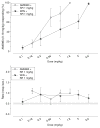
Results: Dose generalization ed50 values increased as a function of the training dose of AM5983, but more so for the partial agonists. The order of potency was (R)-isomer > (±)AM5983 > (S)-isomer and AM5983 > WIN55,212-2 ≥ THC > (R)-(+)-methanandamide. Surmountable antagonism of AM5983 and WIN55,212-2 occurred with rimonabant. The cAMP assay confirmed the cannabinergic nature of AM5983 and CP55,940.
Conclusions: Drug discrimination using different training doses of a high-efficacy, full CB1R agonist differentiated between low- and high-efficacy CB1R agonists.
Conflict of interest statement
Figures

References
-
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. - PubMed
-
- Adams IB, Compton DR, Martin BR. Assessment of anandamide interaction with the cannabinoid brain receptor: SR 141716A antagonism studies in mice and autoradiographic analysis of receptor binding in rat brain. J Pharmacol Exp Ther. 1998;284:1209–1217. - PubMed
-
- Alici T, Appel JB. Increasing the selectivity of the discriminative stimulus effects of delta-9-tetrahydrocannabinol: complete substitution with methanandamide. Pharmacol Biochem Behav. 2004;79:431–437. - PubMed
-
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAAergic ligands. Psychopharmacol (Berl) 2000;153:67–84. - PubMed
-
- Bosier B, Muccioli GG, Hermans E, Lambert DM. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem Pharmacol. 2010;80:1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

