Proteomic profiling of endothelial invasion revealed receptor for activated C kinase 1 (RACK1) complexed with vimentin to regulate focal adhesion kinase (FAK)
- PMID: 24005669
- PMCID: PMC3798542
- DOI: 10.1074/jbc.M113.512467
Proteomic profiling of endothelial invasion revealed receptor for activated C kinase 1 (RACK1) complexed with vimentin to regulate focal adhesion kinase (FAK)
Abstract
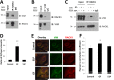
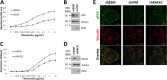
Angiogenesis is critical for many physiological and pathological processes. To identify molecules relevant to angiogenesis, we performed a proteomic screen comparing invading versus non-invading endothelial cells in three-dimensional collagen matrices. We found up-regulated levels of receptor for activated C kinase 1 (RACK1) and the intermediate filament protein vimentin that correlated with increased endothelial cell invasion. Because both RACK1 and vimentin have been linked to focal adhesion kinase (FAK), we investigated whether this pathway regulated invasion. RACK1 depletion reduced invasion responses, and this was associated with attenuated activation of FAK. Knockdown of vimentin significantly decreased levels of phosphorylated and total FAK. Treatment with a pharmacological inhibitor of FAK dose-dependently reduced invasion, indicating a crucial role for FAK activity during invasion. Because RACK1 and vimentin were both up-regulated with sphingosine 1-phosphate treatment, required for invasion, and regulated FAK, we tested whether they complexed together. RACK1 complexed with vimentin, and growth factors enhanced this interaction. In addition, RACK1, vimentin, and FAK formed an intermolecular complex in invading endothelial cultures in three dimensions in response to stimulation by sphingosine 1-phosphate and growth factors. Moreover, depletion of RACK1 decreased the association of vimentin and FAK, suggesting that RACK1 was required for stabilizing vimentin-FAK interactions during sprouting. Silencing of vimentin and RACK1 decreased cell adhesion and focal contact formation. Taken together, these results demonstrate that proangiogenic signals converge to enhance expression and association of RACK1 and vimentin, which regulated FAK, resulting in successful endothelial sprout formation in three-dimensional collagen matrices.
Keywords: Angiogenesis; Collagen; Endothelial Cell; Focal Adhesion Kinase; Morphogenesis; RACK1; Vimentin.
Figures








References
-
- Carmeliet P. (2003) Angiogenesis in health and disease. Nat. Med. 9, 653–660 - PubMed
-
- Folkman J., D'Amore P. A. (1996) Blood vessel formation: what is its molecular basis? Cell 87, 1153–1155 - PubMed
-
- Adams R. H., Alitalo K. (2007) Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 8, 464–478 - PubMed
-
- Kalluri R. (2003) Basement membranes: structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer, 3, 422–433 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

