Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits
- PMID: 24005854
- PMCID: PMC10852667
- DOI: 10.1177/1533317513500839
Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits
Abstract
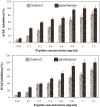
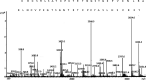
Antioxidant agents and cholinesterase inhibitors are the foremost drugs for the treatment of Alzheimer's disease (AD). In this study, a new peptide from Ziziphus jujuba fruits was investigated for its inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) enzymes as well as antioxidant activity. This peptide was introduced as a new peptide and named Snakin-Z. The Snakin-Z displayed considerable cholinesterase inhibition against AChE and BChE. The half maximal inhibitory concentration (IC50) values of Snakin-Z against AChE and BChE are 0.58 ± 0.08 and 0.72 ± 0.085 mg/mL, respectively. This peptide has 80% enzyme inhibitory activity on AChE and BChE at 1.5 mg/mL. The Snakin-Z also had the high antioxidant activity (IC50 = 0.75 ± 0.09 mg/mL). Thus, it is suggested that Snakin-Z may be beneficial in the treatment of AD. However, more detailed researches are still required as in vivo testing its anticholinesterase and antioxidant activities.
Keywords: Alzheimer’s disease; Ziziphus jujuba fruits; acetylcholinesterase; antioxidant; butyrylcholinesterase.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




Similar articles
-
Investigation of the antimicrobial activities of Snakin-Z, a new cationic peptide derived from Zizyphus jujuba fruits.Nat Prod Res. 2013;27(24):2292-6. doi: 10.1080/14786419.2013.827192. Epub 2013 Aug 20. Nat Prod Res. 2013. PMID: 23962183
-
Bis-Amiridines as Acetylcholinesterase and Butyrylcholinesterase Inhibitors: N-Functionalization Determines the Multitarget Anti-Alzheimer's Activity Profile.Molecules. 2022 Feb 4;27(3):1060. doi: 10.3390/molecules27031060. Molecules. 2022. PMID: 35164325 Free PMC article.
-
Amiridine-piperazine hybrids as cholinesterase inhibitors and potential multitarget agents for Alzheimer's disease treatment.Bioorg Chem. 2021 Jul;112:104974. doi: 10.1016/j.bioorg.2021.104974. Epub 2021 May 21. Bioorg Chem. 2021. PMID: 34029971
-
Management of oxidative stress and other pathologies in Alzheimer's disease.Arch Toxicol. 2019 Sep;93(9):2491-2513. doi: 10.1007/s00204-019-02538-y. Epub 2019 Aug 22. Arch Toxicol. 2019. PMID: 31440798 Review.
-
Cholinesterase Inhibitory Activity of Some semi-Rigid Spiro Heterocycles: POM Analyses and Crystalline Structure of Pharmacophore Site.Mini Rev Med Chem. 2018;18(8):711-716. doi: 10.2174/1389557517666170713114039. Mini Rev Med Chem. 2018. PMID: 28714400 Review.
Cited by
-
Hydrolase-Treated Royal Jelly Attenuates H2O2- and Glutamate-Induced SH-SY5Y Cell Damage and Promotes Cognitive Enhancement in a Rat Model of Vascular Dementia.Int J Food Sci. 2021 Oct 5;2021:2213814. doi: 10.1155/2021/2213814. eCollection 2021. Int J Food Sci. 2021. PMID: 34651043 Free PMC article.
-
Isolation of Bacillus altitudinis 5-DSW with Protease Activity from Deep-Sea Mineral Water and Preparation of Functional Active Peptide Fractions from Chia Seeds.Microorganisms. 2024 Oct 10;12(10):2048. doi: 10.3390/microorganisms12102048. Microorganisms. 2024. PMID: 39458357 Free PMC article.
-
Ziziphus mauritiana Lam. Bark and Leaves: Extraction, Phytochemical Composition, In Vitro Bioassays and In Silico Studies.Plants (Basel). 2024 Aug 8;13(16):2195. doi: 10.3390/plants13162195. Plants (Basel). 2024. PMID: 39204631 Free PMC article.
-
Ziziphus jujuba: Applications in the Pharmacy and Food Industry.Plants (Basel). 2024 Sep 29;13(19):2724. doi: 10.3390/plants13192724. Plants (Basel). 2024. PMID: 39409594 Free PMC article. Review.
-
Bioactive Properties of Enzymatic Gelatin Hydrolysates Based on In Silico, In Vitro, and In Vivo Studies.Molecules. 2024 Sep 16;29(18):4402. doi: 10.3390/molecules29184402. Molecules. 2024. PMID: 39339395 Free PMC article.
References
-
- Krantic S. Peptides as regulators of the immune system: emphasis on somatostatin. Peptides. 2000;21(12):1941–1964. - PubMed
-
- Sung WS, Park Y, Choi CH, Hahm KS, Lee DG. Mode of antibacterial action of a signal peptide, Pep27 from Streptococcus pneumoniae . Biochem Biophys Res Commun. 2007;363(3):806–810. - PubMed
-
- Duval E, Zatylny C, Laurencin M, Baudy-Floc'h M, Henry J. KKKKPLFGLFFGLF: a cationic peptide designed to exert antibacterial activity. Peptides. 2009;30(9):1608–1612. - PubMed
-
- Daoud R, Dubois V, Bors-Dodita L, et al. . New antibacterial peptide derived from bovine hemoglobin. Peptides. 2005;26(5):713–719. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

