Isoform selectivity of adenylyl cyclase inhibitors: characterization of known and novel compounds
- PMID: 24006339
- PMCID: PMC3807061
- DOI: 10.1124/jpet.113.208157
Isoform selectivity of adenylyl cyclase inhibitors: characterization of known and novel compounds
Abstract
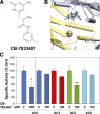
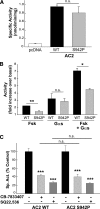
Nine membrane-bound adenylyl cyclase (AC) isoforms catalyze the production of the second messenger cyclic AMP (cAMP) in response to various stimuli. Reduction of AC activity has well documented benefits, including benefits for heart disease and pain. These roles have inspired development of isoform-selective AC inhibitors, a lack of which currently limits exploration of functions and/or treatment of dysfunctions involving AC/cAMP signaling. However, inhibitors described as AC5- or AC1-selective have not been screened against the full panel of AC isoforms. We have measured pharmacological inhibitor profiles for all transmembrane AC isoforms. We found that 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ22,536), 2-amino-7-(furanyl)-7,8-dihydro-5(6H)-quinazolinone (NKY80), and adenine 9-β-d-arabinofuranoside (Ara-A), described as supposedly AC5-selective, do not discriminate between AC5 and AC6, whereas the putative AC1-selective inhibitor 5-[[2-(6-amino-9H-purin-9-yl)ethyl]amino]-1-pentanol (NB001) does not directly target AC1 to reduce cAMP levels. A structure-based virtual screen targeting the ATP binding site of AC was used to identify novel chemical structures that show some preference for AC1 or AC2. Mutation of the AC2 forskolin binding pocket does not interfere with inhibition by SQ22,536 or the novel AC2 inhibitor, suggesting binding to the catalytic site. Thus, we show that compounds lacking the adenine chemical signature and targeting the ATP binding site can potentially be used to develop AC isoform-specific inhibitors, and discuss the need to reinterpret literature using AC5/6-selective molecules SQ22,536, NKY80, and Ara-A.
Figures
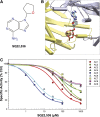
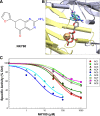
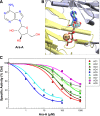
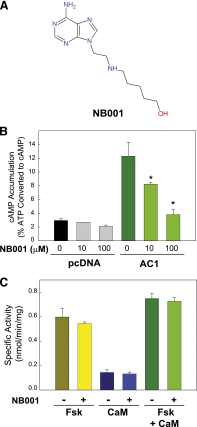

References
-
- Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. (2001) Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ Res 89:997–1004 - PubMed
-
- Bai Y, Tsunematsu T, Jiao Q, Ohnuki Y, Mototani Y, Shiozawa K, Jin M, Cai W, Jin HL, Fujita T, et al. (2012) Pharmacological stimulation of type 5 adenylyl cyclase stabilizes heart rate under both microgravity and hypergravity induced by parabolic flight. J Pharmacol Sci 119:381–389 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

