Atomic structure of Cucumber necrosis virus and the role of the capsid in vector transmission
- PMID: 24006433
- PMCID: PMC3807921
- DOI: 10.1128/JVI.01965-13
Atomic structure of Cucumber necrosis virus and the role of the capsid in vector transmission
Abstract
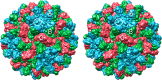
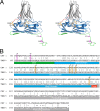
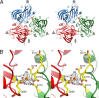
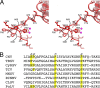
Cucumber Necrosis Virus (CNV) is a member of the genus Tombusvirus and has a monopartite positive-sense RNA genome packaged in a T=3 icosahedral particle. CNV is transmitted in nature via zoospores of the fungus Olpidium bornovanus. CNV undergoes a conformational change upon binding to the zoospore that is required for transmission, and specific polysaccharides on the zoospore surface have been implicated in binding. To better understand this transmission process, we have determined the atomic structure of CNV. As expected, being a member of the Tombusvirus genus, the core structure of CNV is highly similar to that of Tomato bushy stunt virus (TBSV), with major differences lying on the exposed loops. Also, as was seen with TBSV, CNV appears to have a calcium binding site between the subunits around the quasi-3-fold axes. However, unlike TBSV, there appears to be a novel zinc binding site within the β annulus formed by the N termini of the three C subunits at the icosahedral 3-fold axes. Two of the mutations causing defective transmission map immediately around this zinc binding site. The other mutations causing defective transmission and particle formation are mapped onto the CNV structure, and it is likely that a number of the mutations affect zoospore transmission by affecting conformational transitions rather than directly affecting receptor binding.
Figures


References
-
- Rochon DM, Tremaine JH. 1989. Complete nucleotide sequence of the cucumber necrosis virus genome. Virology 169:251–259 - PubMed
-
- Rochon D, Kakani K, Robbins M, Reade R. 2004. Molecular aspects of plant virus transmission by Olpidium and Plasmodiophorid vectors. Annu. Rev. Phytopathol. 42:211–241 - PubMed
-
- Campbell RN. 1996. Fungal transmission of plant viruses. Annu. Rev. Phytopathol. 34:87–108 - PubMed
-
- Dias HF. 1970. The relationship between cucumber necrosis virus by Olpidium cucurbitacaerum. Virology 40:828–839 - PubMed
-
- Harrison SC, Olson AJ, Schutt CE, Winkler FK, Bricogne G. 1978. Tomato bushy stunt virus at 2.9Å resolution. Nature 276:368–373 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

