Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria
- PMID: 24006469
- PMCID: PMC3811607
- DOI: 10.1128/MMBR.00061-12
Functions, compositions, and evolution of the two types of carboxysomes: polyhedral microcompartments that facilitate CO2 fixation in cyanobacteria and some proteobacteria
Abstract
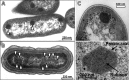
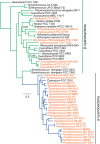
Cyanobacteria are the globally dominant photoautotrophic lineage. Their success is dependent on a set of adaptations collectively termed the CO2-concentrating mechanism (CCM). The purpose of the CCM is to support effective CO2 fixation by enhancing the chemical conditions in the vicinity of the primary CO2-fixing enzyme, D-ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO), to promote the carboxylase reaction and suppress the oxygenase reaction. In cyanobacteria and some proteobacteria, this is achieved by encapsulation of RubisCO within carboxysomes, which are examples of a group of proteinaceous bodies called bacterial microcompartments. Carboxysomes encapsulate the CO2-fixing enzyme within the selectively permeable protein shell and simultaneously encapsulate a carbonic anhydrase enzyme for CO2 supply from a cytoplasmic bicarbonate pool. These bodies appear to have arisen twice and undergone a process of convergent evolution. While the gross structures of all known carboxysomes are ostensibly very similar, with shared gross features such as a selectively permeable shell layer, each type of carboxysome encapsulates a phyletically distinct form of RubisCO enzyme. Furthermore, the specific proteins forming structures such as the protein shell or the inner RubisCO matrix are not identical between carboxysome types. Each type has evolutionarily distinct forms of the same proteins, as well as proteins that are entirely unrelated to one another. In light of recent developments in the study of carboxysome structure and function, we present this review to summarize the knowledge of the structure and function of both types of carboxysome. We also endeavor to cast light on differing evolutionary trajectories which may have led to the differences observed in extant carboxysomes.
Figures







References
-
- Badger MR, Hanson D, Price GD. 2002. Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct. Plant Biol. 29:161–173 - PubMed
-
- Badger MR, Price GD. 2003. CO2 concentrating mechanisms in cyanobacteria: molecular components, their diversity and evolution. J. Exp. Bot. 54:609–622 - PubMed
-
- Raven JA. 2003. Inorganic carbon concentrating mechanisms in relation to the biology of algae. Photosynth. Res. 77:155–171 - PubMed
-
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. 1998. The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can. J. Bot. 76:1052–1071
-
- Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH. 1998. Mechanism of RUBISCO—the carbamate as general base. Chem. Rev. 98:549–561 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

