Mechanism of homologous recombination and implications for aging-related deletions in mitochondrial DNA
- PMID: 24006472
- PMCID: PMC3811608
- DOI: 10.1128/MMBR.00007-13
Mechanism of homologous recombination and implications for aging-related deletions in mitochondrial DNA
Abstract
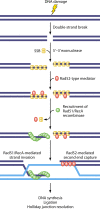
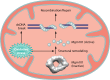
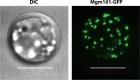
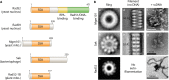
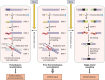
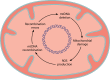
Homologous recombination is a universal process, conserved from bacteriophage to human, which is important for the repair of double-strand DNA breaks. Recombination in mitochondrial DNA (mtDNA) was documented more than 4 decades ago, but the underlying molecular mechanism has remained elusive. Recent studies have revealed the presence of a Rad52-type recombination system of bacteriophage origin in mitochondria, which operates by a single-strand annealing mechanism independent of the canonical RecA/Rad51-type recombinases. Increasing evidence supports the notion that, like in bacteriophages, mtDNA inheritance is a coordinated interplay between recombination, repair, and replication. These findings could have profound implications for understanding the mechanism of mtDNA inheritance and the generation of mtDNA deletions in aging cells.
Figures



Similar articles
-
Presynaptic filament dynamics in homologous recombination and DNA repair.Crit Rev Biochem Mol Biol. 2011 Jun;46(3):240-70. doi: 10.3109/10409238.2011.576007. Crit Rev Biochem Mol Biol. 2011. PMID: 21599536 Free PMC article.
-
Homologous recombination in budding yeast expressing the human RAD52 gene reveals a Rad51-independent mechanism of conservative double-strand break repair.Nucleic Acids Res. 2017 Feb 28;45(4):1879-1888. doi: 10.1093/nar/gkw1228. Nucleic Acids Res. 2017. PMID: 27923995 Free PMC article.
-
Rad51 and Rad52 are involved in homologous recombination of replicating herpes simplex virus DNA.PLoS One. 2014 Nov 3;9(11):e111584. doi: 10.1371/journal.pone.0111584. eCollection 2014. PLoS One. 2014. PMID: 25365323 Free PMC article.
-
DNA Repair: The Search for Homology.Bioessays. 2018 May;40(5):e1700229. doi: 10.1002/bies.201700229. Epub 2018 Mar 30. Bioessays. 2018. PMID: 29603285 Free PMC article. Review.
-
Repair of DNA double-strand breaks in plant meiosis: role of eukaryotic RecA recombinases and their modulators.Plant Reprod. 2023 Mar;36(1):17-41. doi: 10.1007/s00497-022-00443-6. Epub 2022 Jun 1. Plant Reprod. 2023. PMID: 35641832 Review.
Cited by
-
Genetic instability in budding and fission yeast-sources and mechanisms.FEMS Microbiol Rev. 2015 Nov;39(6):917-67. doi: 10.1093/femsre/fuv028. Epub 2015 Jun 24. FEMS Microbiol Rev. 2015. PMID: 26109598 Free PMC article. Review.
-
Mitochondria-cytosol-nucleus crosstalk: learning from Saccharomyces cerevisiae.FEMS Yeast Res. 2018 Dec 1;18(8):foy088. doi: 10.1093/femsyr/foy088. FEMS Yeast Res. 2018. PMID: 30165482 Free PMC article. Review.
-
Deletion of OGG1 Results in a Differential Signature of Oxidized Purine Base Damage in mtDNA Regions.Int J Mol Sci. 2019 Jul 5;20(13):3302. doi: 10.3390/ijms20133302. Int J Mol Sci. 2019. PMID: 31284385 Free PMC article.
-
Evolution and maintenance of mtDNA gene content across eukaryotes.Biochem J. 2024 Aug 7;481(15):1015-1042. doi: 10.1042/BCJ20230415. Biochem J. 2024. PMID: 39101615 Free PMC article. Review.
-
A novel fragmented mitochondrial genome in the protist pathogen Toxoplasma gondii and related tissue coccidia.Genome Res. 2021 May;31(5):852-865. doi: 10.1101/gr.266403.120. Epub 2021 Apr 27. Genome Res. 2021. PMID: 33906963 Free PMC article.
References
-
- Wallace DC. 2007. Why do we still have a maternally inherited mitochondrial DNA? Insights from evolutionary medicine. Annu. Rev. Biochem. 76:781–821 - PubMed
-
- Chen XJ, Butow RA. 2005. The organization and inheritance of the mitochondrial genome. Nat. Rev. Genet. 6:815–825 - PubMed
-
- Holt IJ, He J, Mao CC, Boyd-Kirkup JD, Martinsson P, Sembongi H, Reyes A, Spelbrink JN. 2007. Mammalian mitochondrial nucleoids: organizing an independently minded genome. Mitochondrion 7:311–321 - PubMed
-
- Bogenhagen DF. 2011. Mitochondrial DNA nucleoid structure. Biochim. Biophys. Acta 1819:914–920 - PubMed
-
- Spelbrink JN. 2010. Functional organization of mammalian mitochondrial DNA in nucleoids: history, recent developments, and future challenges. IUBMB Life 62:19–32 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

