What a difference a Dalton makes: bacterial virulence factors modulate eukaryotic host cell signaling systems via deamidation
- PMID: 24006474
- PMCID: PMC3811613
- DOI: 10.1128/MMBR.00013-13
What a difference a Dalton makes: bacterial virulence factors modulate eukaryotic host cell signaling systems via deamidation
Abstract
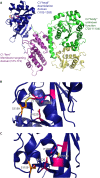
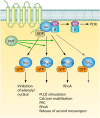
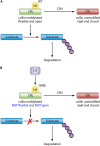
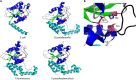
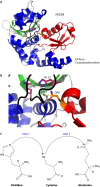
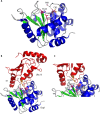
Pathogenic bacteria commonly deploy enzymes to promote virulence. These enzymes can modulate the functions of host cell targets. While the actions of some enzymes can be very obvious (e.g., digesting plant cell walls), others have more subtle activities. Depending on the lifestyle of the bacteria, these subtle modifications can be crucially important for pathogenesis. In particular, if bacteria rely on a living host, subtle mechanisms to alter host cellular function are likely to dominate. Several bacterial virulence factors have evolved to use enzymatic deamidation as a subtle posttranslational mechanism to modify the functions of host protein targets. Deamidation is the irreversible conversion of the amino acids glutamine and asparagine to glutamic acid and aspartic acid, respectively. Interestingly, all currently characterized bacterial deamidases affect the function of the target protein by modifying a single glutamine residue in the sequence. Deamidation of target host proteins can disrupt host signaling and downstream processes by either activating or inactivating the target. Despite the subtlety of this modification, it has been shown to cause dramatic, context-dependent effects on host cells. Several crystal structures of bacterial deamidases have been solved. All are members of the papain-like superfamily and display a cysteine-based catalytic triad. However, these proteins form distinct structural subfamilies and feature combinations of modular domains of various functions. Based on the diverse pathogens that use deamidation as a mechanism to promote virulence and the recent identification of multiple deamidases, it is clear that this enzymatic activity is emerging as an important and widespread feature in bacterial pathogenesis.
Figures





References
-
- Cui J, Shao F. 2011. Biochemistry and cell signaling taught by bacterial effectors. Trends Biochem. Sci. 36:532–540 - PubMed
-
- Yarbrough ML, Orth K. 2009. AMPylation is a new post-translational modiFICation. Nat. Chem. Biol. 5:378–379 - PubMed
-
- Robinson AB, Rudd CJ. 1974. Deamidation of glutaminyl and asparaginyl residues in peptides and proteins. Curr. Top. Cell. Regul. 8:247–295 - PubMed
-
- Chao X, Muff TJ, Park SY, Zhang S, Pollard AM, Ordal GW, Bilwes AM, Crane BR. 2006. A receptor-modifying deamidase in complex with a signaling phosphatase reveals reciprocal regulation. Cell 124:561–571 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/J/000C0624/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- HHMI/Howard Hughes Medical Institute/United States
- BB/J004553/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F008732/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources

