Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia
- PMID: 24006476
- PMCID: PMC3869357
- DOI: 10.1093/hmg/ddt429
Multilineage somatic activating mutations in HRAS and NRAS cause mosaic cutaneous and skeletal lesions, elevated FGF23 and hypophosphatemia
Abstract
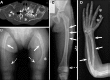
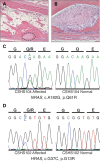
Pathologically elevated serum levels of fibroblast growth factor-23 (FGF23), a bone-derived hormone that regulates phosphorus homeostasis, result in renal phosphate wasting and lead to rickets or osteomalacia. Rarely, elevated serum FGF23 levels are found in association with mosaic cutaneous disorders that affect large proportions of the skin and appear in patterns corresponding to the migration of ectodermal progenitors. The cause and source of elevated serum FGF23 is unknown. In those conditions, such as epidermal and large congenital melanocytic nevi, skin lesions are variably associated with other abnormalities in the eye, brain and vasculature. The wide distribution of involved tissues and the appearance of multiple segmental skin and bone lesions suggest that these conditions result from early embryonic somatic mutations. We report five such cases with elevated serum FGF23 and bone lesions, four with large epidermal nevi and one with a giant congenital melanocytic nevus. Exome sequencing of blood and affected skin tissue identified somatic activating mutations of HRAS or NRAS in each case without recurrent secondary mutation, and we further found that the same mutation is present in dysplastic bone. Our finding of somatic activating RAS mutation in bone, the endogenous source of FGF23, provides the first evidence that elevated serum FGF23 levels, hypophosphatemia and osteomalacia are associated with pathologic Ras activation and may provide insight in the heretofore limited understanding of the regulation of FGF23.
Figures



Similar articles
-
Postzygotic HRAS mutation causing both keratinocytic epidermal nevus and thymoma and associated with bone dysplasia and hypophosphatemia due to elevated FGF23.J Clin Endocrinol Metab. 2014 Jan;99(1):E132-6. doi: 10.1210/jc.2013-2813. Epub 2013 Dec 20. J Clin Endocrinol Metab. 2014. PMID: 24243633
-
Cutaneous skeletal hypophosphatemia syndrome (CSHS) is a multilineage somatic mosaic RASopathy.J Am Acad Dermatol. 2016 Aug;75(2):420-7. doi: 10.1016/j.jaad.2015.11.012. J Am Acad Dermatol. 2016. PMID: 27444071 Free PMC article. Review.
-
Murine models of HRAS-mediated cutaneous skeletal hypophosphatemia syndrome suggest bone as the FGF23 excess source.J Clin Invest. 2023 May 1;133(9):e159330. doi: 10.1172/JCI159330. J Clin Invest. 2023. PMID: 36943390 Free PMC article.
-
Cutaneous skeletal hypophosphatemia syndrome: clinical spectrum, natural history, and treatment.Osteoporos Int. 2016 Dec;27(12):3615-3626. doi: 10.1007/s00198-016-3702-8. Epub 2016 Aug 6. Osteoporos Int. 2016. PMID: 27497815 Free PMC article. Review.
-
Mosaic NRAS Q61R mutation in a child with giant congenital melanocytic naevus, epidermal naevus syndrome and hypophosphataemic rickets.Clin Exp Dermatol. 2017 Jan;42(1):75-79. doi: 10.1111/ced.12969. Epub 2016 Nov 30. Clin Exp Dermatol. 2017. PMID: 27900779
Cited by
-
Heritable and acquired disorders of phosphate metabolism: Etiologies involving FGF23 and current therapeutics.Bone. 2017 Sep;102:31-39. doi: 10.1016/j.bone.2017.01.034. Epub 2017 Jan 31. Bone. 2017. PMID: 28159712 Free PMC article. Review.
-
A case report of mesenchymal scapular FGF secreting tumor: Importance of follow up in tumor induced osteomalacia.Radiol Case Rep. 2021 Feb 17;16(4):989-993. doi: 10.1016/j.radcr.2021.02.002. eCollection 2021 Apr. Radiol Case Rep. 2021. PMID: 33664928 Free PMC article.
-
The Causes of Hypo- and Hyperphosphatemia in Humans.Calcif Tissue Int. 2021 Jan;108(1):41-73. doi: 10.1007/s00223-020-00664-9. Epub 2020 Apr 13. Calcif Tissue Int. 2021. PMID: 32285168 Review.
-
Expansion of the complex genotypic and phenotypic spectrum of FGFR2-associated neurocutaneous syndromes.Hum Genet. 2024 Feb;143(2):159-168. doi: 10.1007/s00439-023-02634-1. Epub 2024 Jan 24. Hum Genet. 2024. PMID: 38265560 Free PMC article.
-
Somatic Mutations in NEK9 Cause Nevus Comedonicus.Am J Hum Genet. 2016 May 5;98(5):1030-1037. doi: 10.1016/j.ajhg.2016.03.019. Am J Hum Genet. 2016. PMID: 27153399 Free PMC article.
References
-
- Blaschko A. Die Nervenverteilung in der Haut in ihrer Beziehung zu den Erkrankungen der Haut. Beilage zu den Verhandlungen der Deutschen Dermatologischen Gesellschaft VII Congress. Breslau, Braumuller, Wien, 1901. 1901
-
- Happle R. The McCune-Albright syndrome: a lethal gene surviving by mosaicism. Clin. Genet. 1986;29:321–324. - PubMed
-
- Rieger E., Kofler R., Borkenstein M., Schwingshandl J., Soyer H.P., Kerl H. Melanotic macules following Blaschko's lines in McCune-Albright syndrome. Br. J. Dermatol. 1994;130:215–220. - PubMed
-
- Tannous Z.S., Mihm M.C., Jr., Sober A.J., Duncan L.M. Congenital melanocytic nevi: clinical and histopathologic features, risk of melanoma, and clinical management. J. Am. Acad. Dermatol. 2005;52:197–203. - PubMed
-
- Vujevich J.J., Mancini A.J. The epidermal nevus syndromes: multisystem disorders. J. Am. Acad. Dermatol. 2004;50:957–961. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

