CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions
- PMID: 24006483
- PMCID: PMC3814154
- DOI: 10.1091/mbc.E13-01-0063
CD47 plays a critical role in T-cell recruitment by regulation of LFA-1 and VLA-4 integrin adhesive functions
Abstract
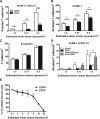
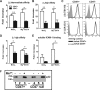
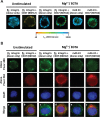
CD47 plays an important but incompletely understood role in the innate and adaptive immune responses. CD47, also called integrin-associated protein, has been demonstrated to associate in cis with β1 and β3 integrins. Here we test the hypothesis that CD47 regulates adhesive functions of T-cell α4β1 (VLA-4) and αLβ2 (LFA-1) in in vivo and in vitro models of inflammation. Intravital microscopy studies reveal that CD47(-/-) Th1 cells exhibit reduced interactions with wild-type (WT) inflamed cremaster muscle microvessels. Similarly, murine CD47(-/-) Th1 cells, as compared with WT, showed defects in adhesion and transmigration across tumor necrosis factor-α (TNF-α)-activated murine endothelium and in adhesion to immobilized intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion protein 1 (VCAM-1) under flow conditions. Human Jurkat T-cells lacking CD47 also showed reduced adhesion to TNF-α-activated endothelium and ICAM-1 and VCAM-1. In cis interactions between Jurkat T-cell β2 integrins and CD47 were detected by fluorescence lifetime imaging microscopy. Unexpectedly, Jurkat CD47 null cells exhibited a striking defect in β1 and β2 integrin activation in response to Mn(2+) or Mg(2+)/ethylene glycol tetraacetic acid treatment. Our results demonstrate that CD47 associates with β2 integrins and is necessary to induce high-affinity conformations of LFA-1 and VLA-4 that recognize their endothelial cell ligands and support leukocyte adhesion and transendothelial migration.
Figures
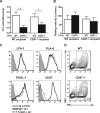
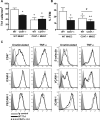
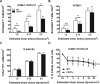
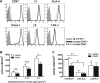
Similar articles
-
Defects in CD4+ T cell LFA-1 integrin-dependent adhesion and proliferation protect Cd47-/- mice from EAE.J Leukoc Biol. 2017 Feb;101(2):493-505. doi: 10.1189/jlb.3A1215-546RR. Epub 2016 Dec 13. J Leukoc Biol. 2017. PMID: 27965383 Free PMC article.
-
Novel role of CD47 in rat microvascular endothelium: signaling and regulation of T-cell transendothelial migration.Arterioscler Thromb Vasc Biol. 2013 Nov;33(11):2566-76. doi: 10.1161/ATVBAHA.113.301903. Epub 2013 Aug 29. Arterioscler Thromb Vasc Biol. 2013. PMID: 23990210 Free PMC article.
-
TSP-1-CD47-integrin α4β1 axis drives T cell infiltration and synovial inflammation in rheumatoid arthritis.Front Immunol. 2025 Apr 16;16:1524304. doi: 10.3389/fimmu.2025.1524304. eCollection 2025. Front Immunol. 2025. PMID: 40308591 Free PMC article.
-
Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases.Med Res Rev. 2002 Mar;22(2):146-67. doi: 10.1002/med.10001. Med Res Rev. 2002. PMID: 11857637 Review.
-
The roles of adhesion molecules and proteinases in lymphocyte transendothelial migration.Biochem Cell Biol. 1996;74(6):749-57. doi: 10.1139/o96-082. Biochem Cell Biol. 1996. PMID: 9164645 Review.
Cited by
-
CD47 expression is critical for CAR T-cell survival in vivo.J Immunother Cancer. 2023 Mar;11(3):e005857. doi: 10.1136/jitc-2022-005857. J Immunother Cancer. 2023. PMID: 36918226 Free PMC article.
-
Human lung adenocarcinoma CD47 is upregulated by interferon-γ and promotes tumor metastasis.Mol Ther Oncolytics. 2022 May 3;25:276-287. doi: 10.1016/j.omto.2022.04.011. eCollection 2022 Jun 16. Mol Ther Oncolytics. 2022. PMID: 35663227 Free PMC article.
-
CD47 antibody blockade suppresses microglia-dependent phagocytosis and monocyte transition to macrophages, impairing recovery in EAE.JCI Insight. 2021 Nov 8;6(21):e148719. doi: 10.1172/jci.insight.148719. JCI Insight. 2021. PMID: 34591795 Free PMC article.
-
Editorial Commentary: Top Five Stories of the Cellular Landscape and Therapies of Atherosclerosis: Current Knowledge and Future Perspectives.Curr Med Sci. 2024 Feb;44(1):241-243. doi: 10.1007/s11596-023-2825-3. Curr Med Sci. 2024. PMID: 38277018 No abstract available.
-
A DOCK8-WIP-WASp complex links T cell receptors to the actin cytoskeleton.J Clin Invest. 2016 Oct 3;126(10):3837-3851. doi: 10.1172/JCI85774. Epub 2016 Sep 6. J Clin Invest. 2016. PMID: 27599296 Free PMC article.
References
-
- Andrew D, Shock A, Ball E, Ortlepp S, Bell J, Robinson M. KIM185, a monoclonal antibody to CD18 which induces a change in the conformation of CD18 and promotes both LFA-1- and CR3-dependent adhesion. Eur J Immunol. 1993;23:2217–2222. - PubMed
-
- Azcutia V, Stefanidakis M, Tsuboi N, Mayadas T, Croce KJ, Fukuda D, Aikawa M, Newton G, Luscinskas FW. Endothelial CD47 promotes vascular endothelial-cadherin tyrosine phosphorylation and participates in T cell recruitment at sites of inflammation in vivo. J Immunol. 2012;189:2553–2563. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- S10 RR027931/RR/NCRR NIH HHS/United States
- R01 AI068871-04S1/AI/NIAID NIH HHS/United States
- P01 HL36028/HL/NHLBI NIH HHS/United States
- K01 DK089145-01A1/DK/NIDDK NIH HHS/United States
- P01 HL036028/HL/NHLBI NIH HHS/United States
- K01 DK089145/DK/NIDDK NIH HHS/United States
- 1S10RR027931/RR/NCRR NIH HHS/United States
- T32 DK007540-22/DK/NIDDK NIH HHS/United States
- 1R01HL097796/HL/NHLBI NIH HHS/United States
- K08 HL086672/HL/NHLBI NIH HHS/United States
- R01 DK072564/DK/NIDDK NIH HHS/United States
- R01 AI068871/AI/NIAID NIH HHS/United States
- R01 HL097796/HL/NHLBI NIH HHS/United States
- T32 DK007540/DK/NIDDK NIH HHS/United States
- HL53192/HL/NHLBI NIH HHS/United States
- F32 HL105016/HL/NHLBI NIH HHS/United States
- R01 DK079392/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

