Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β
- PMID: 24006485
- PMCID: PMC3814150
- DOI: 10.1091/mbc.E12-10-0776
Targeted inactivation of β1 integrin induces β3 integrin switching, which drives breast cancer metastasis by TGF-β
Erratum in
- Mol Biol Cell. 2014 Feb;25(4):548
Abstract
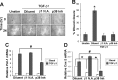
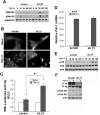
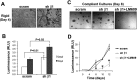
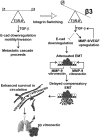
Mammary tumorigenesis and epithelial-mesenchymal transition (EMT) programs cooperate in converting transforming growth factor-β (TGF-β) from a suppressor to a promoter of breast cancer metastasis. Although previous reports associated β1 and β3 integrins with TGF-β stimulation of EMT and metastasis, the functional interplay and plasticity exhibited by these adhesion molecules in shaping the oncogenic activities of TGF-β remain unknown. We demonstrate that inactivation of β1 integrin impairs TGF-β from stimulating the motility of normal and malignant mammary epithelial cells (MECs) and elicits robust compensatory expression of β3 integrin solely in malignant MECs, but not in their normal counterparts. Compensatory β3 integrin expression also 1) enhances the growth of malignant MECs in rigid and compliant three-dimensional organotypic cultures and 2) restores the induction of the EMT phenotypes by TGF-β. Of importance, compensatory expression of β3 integrin rescues the growth and pulmonary metastasis of β1 integrin-deficient 4T1 tumors in mice, a process that is prevented by genetic depletion or functional inactivation of β3 integrin. Collectively our findings demonstrate that inactivation of β1 integrin elicits metastatic progression via a β3 integrin-specific mechanism, indicating that dual β1 and β3 integrin targeting is necessary to alleviate metastatic disease in breast cancer patients.
Figures




Similar articles
-
Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis.Breast Cancer Res. 2009;11(5):R68. doi: 10.1186/bcr2360. Breast Cancer Res. 2009. PMID: 19740433 Free PMC article.
-
Beta3 integrin and Src facilitate transforming growth factor-beta mediated induction of epithelial-mesenchymal transition in mammary epithelial cells.Breast Cancer Res. 2006;8(4):R42. doi: 10.1186/bcr1524. Breast Cancer Res. 2006. PMID: 16859511 Free PMC article.
-
Upregulated WAVE3 expression is essential for TGF-β-mediated EMT and metastasis of triple-negative breast cancer cells.Breast Cancer Res Treat. 2013 Nov;142(2):341-53. doi: 10.1007/s10549-013-2753-1. Epub 2013 Nov 7. Breast Cancer Res Treat. 2013. PMID: 24197660 Free PMC article.
-
The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells.J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):169-90. doi: 10.1007/s10911-010-9181-1. Epub 2010 May 15. J Mammary Gland Biol Neoplasia. 2010. PMID: 20467795 Free PMC article. Review.
-
Noncanonical TGF-β signaling during mammary tumorigenesis.J Mammary Gland Biol Neoplasia. 2011 Jun;16(2):127-46. doi: 10.1007/s10911-011-9207-3. Epub 2011 Mar 31. J Mammary Gland Biol Neoplasia. 2011. PMID: 21448580 Free PMC article. Review.
Cited by
-
TGF-β signaling: critical nexus of fibrogenesis and cancer.J Transl Med. 2024 Jun 26;22(1):594. doi: 10.1186/s12967-024-05411-4. J Transl Med. 2024. PMID: 38926762 Free PMC article. Review.
-
Regulation of inside-out β1-integrin activation by CDCP1.Oncogene. 2018 May;37(21):2817-2836. doi: 10.1038/s41388-018-0142-2. Epub 2018 Mar 7. Oncogene. 2018. PMID: 29511352 Free PMC article.
-
Tuning Collective Cell Migration by Cell-Cell Junction Regulation.Cold Spring Harb Perspect Biol. 2017 Apr 3;9(4):a029199. doi: 10.1101/cshperspect.a029199. Cold Spring Harb Perspect Biol. 2017. PMID: 28096261 Free PMC article. Review.
-
Biphasic α2β1 Integrin Expression in Breast Cancer Metastasis to Bone.Int J Mol Sci. 2021 Jun 27;22(13):6906. doi: 10.3390/ijms22136906. Int J Mol Sci. 2021. PMID: 34199096 Free PMC article.
-
Ursolic Acid Attenuates TGF-β1-Induced Epithelial-Mesenchymal Transition in NSCLC by Targeting Integrin αVβ5/MMPs Signaling.Oncol Res. 2019 May 7;27(5):593-600. doi: 10.3727/096504017X15051723858706. Epub 2017 Sep 14. Oncol Res. 2019. PMID: 28911340 Free PMC article.
References
-
- Bei L, Lu Y, Bellis SL, Zhou W, Horvath E, Eklund EA. Identification of a HoxA10 activation domain necessary for transcription of the gene encoding b3 integrin during myeloid differentiation. J Biol Chem. 2007;282:16846–16859. - PubMed
-
- Ben-Ze'ev A. The dual role of cytoskeletal anchor proteins in cell adhesion and signal transduction. Ann NY Acad Sci. 1999;886:37–47. - PubMed
-
- Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin b1 signaling is necessary for transforming growth factor-b activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

