Differential topical susceptibility to TGFβ in intact and injured regions of the epithelium: key role in myofibroblast transition
- PMID: 24006486
- PMCID: PMC3814143
- DOI: 10.1091/mbc.E13-04-0220
Differential topical susceptibility to TGFβ in intact and injured regions of the epithelium: key role in myofibroblast transition
Abstract
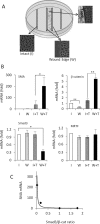
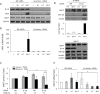
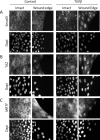
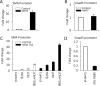
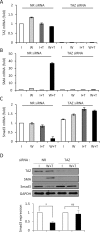
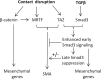
Induction of epithelial-myofibroblast transition (EMyT), a robust fibrogenic phenotype change hallmarked by α-smooth muscle actin (SMA) expression, requires transforming growth factor-β1 (TGFβ) and the absence/uncoupling of intracellular contacts. This suggests that an "injured" epithelium may be topically susceptible to TGFβ. To explore this concept, we use an epithelial wound model in which intact and contact-deprived regions of the same monolayer can be analyzed simultaneously. We show that TGFβ elicits dramatically different responses at these two loci. SMA expression and initially enhanced nuclear Smad3 accumulation followed by Smad3 mRNA and protein down-regulation occur exclusively at the wound. Mechanistically, three transcriptional coactivators whose localization is regulated by cell contact integrity are critical for these local effects. These are myocardin-related transcription factor (MRTF), the driver of the SMA promoter; β-catenin, which counteracts the known inhibitory effect of Smad3 on MRTF and maintains MRTF protein stability and mRNA expression in the wound; and TAZ, a Hippo effector and Smad3 retention factor. Remarkably, active TAZ stimulates the SMA and suppresses the Smad3 promoter, whereas TAZ silencing prevents wound-restricted expression of SMA and loss of Smad3. Such locus-specific reprogramming might play key roles in wound healing and the susceptibility of the injured epithelium to fibrogenesis.
Figures

Similar articles
-
TGF-β1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism.J Biol Chem. 2017 Sep 8;292(36):14902-14920. doi: 10.1074/jbc.M117.780502. Epub 2017 Jul 24. J Biol Chem. 2017. PMID: 28739802 Free PMC article.
-
β-catenin and Smad3 regulate the activity and stability of myocardin-related transcription factor during epithelial-myofibroblast transition.Mol Biol Cell. 2011 Dec;22(23):4472-85. doi: 10.1091/mbc.E11-04-0335. Epub 2011 Sep 30. Mol Biol Cell. 2011. PMID: 21965288 Free PMC article.
-
Myocardin-related Transcription Factor Regulates Nox4 Protein Expression: LINKING CYTOSKELETAL ORGANIZATION TO REDOX STATE.J Biol Chem. 2016 Jan 1;291(1):227-43. doi: 10.1074/jbc.M115.674606. Epub 2015 Nov 10. J Biol Chem. 2016. PMID: 26555261 Free PMC article.
-
Astaxanthin prevents TGFβ1-induced pro-fibrogenic gene expression by inhibiting Smad3 activation in hepatic stellate cells.Biochim Biophys Acta. 2015 Jan;1850(1):178-85. doi: 10.1016/j.bbagen.2014.10.014. Epub 2014 Oct 23. Biochim Biophys Acta. 2015. PMID: 25450180
-
Smaddening complexity: the role of Smad3 in epithelial-myofibroblast transition.Cells Tissues Organs. 2011;193(1-2):41-52. doi: 10.1159/000320180. Epub 2010 Nov 3. Cells Tissues Organs. 2011. PMID: 21051861 Review.
Cited by
-
Mediated nuclear import and export of TAZ and the underlying molecular requirements.Nat Commun. 2018 Nov 23;9(1):4966. doi: 10.1038/s41467-018-07450-0. Nat Commun. 2018. PMID: 30470756 Free PMC article.
-
Anti-microRNA-378a enhances wound healing process by upregulating integrin beta-3 and vimentin.Mol Ther. 2014 Oct;22(10):1839-50. doi: 10.1038/mt.2014.115. Epub 2014 Jun 23. Mol Ther. 2014. PMID: 24954475 Free PMC article.
-
Biomechanics of TGFβ-induced epithelial-mesenchymal transition: implications for fibrosis and cancer.Clin Transl Med. 2014 Jul 15;3:23. doi: 10.1186/2001-1326-3-23. eCollection 2014. Clin Transl Med. 2014. PMID: 25097726 Free PMC article. Review.
-
TGF-β1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism.J Biol Chem. 2017 Sep 8;292(36):14902-14920. doi: 10.1074/jbc.M117.780502. Epub 2017 Jul 24. J Biol Chem. 2017. PMID: 28739802 Free PMC article.
-
Evasion of apoptosis by myofibroblasts: a hallmark of fibrotic diseases.Nat Rev Rheumatol. 2020 Jan;16(1):11-31. doi: 10.1038/s41584-019-0324-5. Epub 2019 Dec 2. Nat Rev Rheumatol. 2020. PMID: 31792399 Free PMC article. Review.
References
-
- Ashcroft GS, et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol. 1999;1:260–266. - PubMed
-
- Busche S, Descot A, Julien S, Genth H, Posern G. Epithelial cell-cell contacts regulate SRF-mediated transcription via Rac-actin-MAL signalling. J Cell Sci. 2008;121:1025–1035. - PubMed
-
- Chapman HA. Epithelial-mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

