Novel ex vivo culture method for human monocytes uses shear flow to prevent total loss of transendothelial diapedesis function
- PMID: 24006509
- PMCID: PMC3868191
- DOI: 10.1189/jlb.0513272
Novel ex vivo culture method for human monocytes uses shear flow to prevent total loss of transendothelial diapedesis function
Abstract
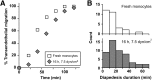
Monocyte recruitment to inflammatory sites and their transendothelial migration into tissues are critical to homeostasis and pathogenesis of chronic inflammatory diseases. However, even short-term suspension culture of primary human monocytes leads to phenotypic changes. In this study, we characterize the functional effects of ex vivo monocyte culture on the steps involved in monocyte transendothelial migration. Our data demonstrate that monocyte diapedesis is impaired by as little as 4 h culture, and the locomotion step is subsequently compromised. After 16 h in culture, monocyte diapedesis is irreversibly reduced by ∼90%. However, maintenance of monocytes under conditions mimicking physiological flow (5-7.5 dyn/cm²) is sufficient to reduce diapedesis impairment significantly. Thus, through the application of shear during ex vivo culture of monocytes, our study establishes a novel protocol, allowing functional analyses of monocytes not currently possible under static culture conditions. These data further suggest that monocyte-based therapeutic applications may be measurably improved by alteration of ex vivo conditions before their use in patients.
Keywords: macrophages; migration; transfusion medicine; vascular biology.
Figures



References
-
- Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. (2007) Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678–689 - PubMed
-
- Randolph G. J., Beaulieu S., Lebecque S., Steinman R. M., Muller W. A. (1998) Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science 282, 480–483 - PubMed
-
- Muller W. A., Randolph G. J. (1999) Migration of leukocytes across endothelium and beyond: molecules involved in the transmigration and fate of monocytes. J. Leukoc. Biol. 66, 698–704 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

