Simultaneous identification of 36 mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA in a single reaction by multiplex assay kit
- PMID: 24006859
- PMCID: PMC3844320
- DOI: 10.1186/1471-2407-13-405
Simultaneous identification of 36 mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA in a single reaction by multiplex assay kit
Abstract
Background: Retrospective analyses in the West suggest that mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA are negative predictive factors for cetuximab treatment in colorectal cancer patients. We developed a novel multiplex kit detecting 36 mutations in KRAS codons 61 and 146, BRAF, NRAS, and PIK3CA using Luminex (xMAP) assay in a single reaction.
Methods: Tumor samples and clinical data from Asian colorectal cancer patients treated with cetuximab were collected. We investigated KRAS, BRAF, NRAS, and PIK3CA mutations using both the multiplex kit and direct sequencing methods, and evaluated the concordance between the 2 methods. Objective response, progression-free survival (PFS), and overall survival (OS) were also evaluated according to mutational status.
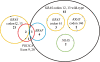
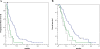
Results: In total, 82 of 83 samples (78 surgically resected specimens and 5 biopsy specimens) were analyzed using both methods. All multiplex assays were performed using 50 ng of template DNA. The concordance rate between the methods was 100%. Overall, 49 (59.8%) patients had all wild-type tumors, 21 (25.6%) had tumors harboring KRAS codon 12 or 13 mutations, and 12 (14.6%) had tumors harboring KRAS codon 61, KRAS codon 146, BRAF, NRAS, or PIK3CA mutations. The response rates in these patient groups were 38.8%, 4.8%, and 0%, respectively. Median PFS in these groups was 6.1 months (95% confidence interval (CI): 3.1-9.2), 2.7 months (1.2-4.2), and 1.6 months (1.5-1.7); median OS was 13.8 months (9.2-18.4), 8.2 months (5.7-10.7), and 6.3 months (1.3-11.3), respectively. Statistically significant differences in both PFS and OS were found between patients with all wild-type tumors and those with KRAS codon 61, KRAS codon 146, BRAF, NRAS, or PIK3CA mutations (PFS: 95% CI, 0.11-0.44; P < 0.0001; OS: 95% CI, 0.15-0.61; P < 0.0001).
Conclusions: Our newly developed multiplex kit is practical and feasible for investigation of a range of sample types. Moreover, mutations in KRAS codon 61, KRAS codon 146, BRAF, NRAS, or PIK3CA detected in Asian patients were not predictive of clinical benefits from cetuximab treatment, similar to the result obtained in European studies.
Figures
References
-
- Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. et al.Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28(31):4697–4705. doi: 10.1200/JCO.2009.27.4860. - DOI - PubMed
-
- Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, Andre T, Chan E, Lordick F, Punt CJ. et al.Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28(31):4706–4713. doi: 10.1200/JCO.2009.27.6055. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

