Contribution of cytoskeletal elements to the axonal mechanical properties
- PMID: 24007256
- PMCID: PMC3846871
- DOI: 10.1186/1754-1611-7-21
Contribution of cytoskeletal elements to the axonal mechanical properties
Abstract
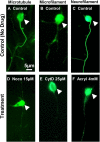
Background: Microtubules, microfilaments, and neurofilaments are cytoskeletal elements that affect cell morphology, cellular processes, and mechanical structures in neural cells. The objective of the current study was to investigate the contribution of each type of cytoskeletal element to the mechanical properties of axons of dorsal root and sympathetic ganglia cells in chick embryos.
Results: Microtubules, microfilaments, and neurofilaments in axons were disrupted by nocodazole, cytochalasin D, and acrylamide, respectively, or a combination of the three. An atomic force microscope (AFM) was then used to compress the treated axons, and the resulting corresponding force-deformation information was analyzed to estimate the mechanical properties of axons that were partially or fully disrupted.
Conclusion: We have found that the mechanical stiffness was most reduced in microtubules-disrupted-axons, followed by neurofilaments-disrupted- and microfilaments-disrupted-axons. This suggests that microtubules contribute the most of the mechanical stiffness to axons.
Figures





References
-
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

