Detailed expression analysis of regulatory genes in the early developing human neural tube
- PMID: 24007338
- PMCID: PMC3870486
- DOI: 10.1089/scd.2013.0309
Detailed expression analysis of regulatory genes in the early developing human neural tube
Abstract
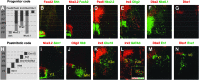
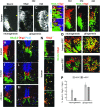
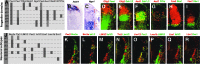
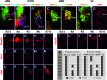
Studies in model organisms constitute the basis of our understanding of the principal molecular mechanisms of cell fate determination in the developing central nervous system. Considering the emergent applications in stem cell-based regenerative medicine, it is important to demonstrate conservation of subtype specific gene expression programs in human as compared to model vertebrates. We have examined the expression patterns of key regulatory genes in neural progenitor cells and their neuronal and glial descendants in the developing human spinal cord, hindbrain, and midbrain, and compared these with developing mouse and chicken embryos. As anticipated, gene expression patterns are highly conserved between these vertebrate species, but there are also features that appear unique to human development. In particular, we find that neither tyrosine hydroxylase nor Nurr1 are specific markers for mesencephalic dopamine neurons, as these genes also are expressed in other neuronal subtypes in the human ventral midbrain and in human embryonic stem cell cultures directed to differentiate towards a ventral mesencephalic identity. Moreover, somatic motor neurons in the ventral spinal cord appear to be produced by two molecularly distinct ventral progenitor populations in the human, raising the possibility that the acquisition of unique ventral progenitor identities may have contributed to the emergence of neural subtypes in higher vertebrates.
Figures

References
-
- Arenas E. (2010). Towards stem cell replacement therapies for Parkinson's disease. Biochem Biophys Res Commun 396:152–156 - PubMed
-
- Chipman PH, Toma JS. and Rafuse VF. (2012). Generation of motor neurons from pluripotent stem cells. Prog Brain Res 201:313–331 - PubMed
-
- Panman L, Andersson E, Alekseenko Z, Hedlund E, Kee N, Mong J, Uhde CW, Deng Q, Sandberg R, et al. (2011). Transcription factor-induced lineage selection of stem-cell-derived neural progenitor cells. Cell Stem Cell 8:663–675 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

