Mesenchymal stem cell transplantation for the infarcted heart: therapeutic potential for insulin resistance beyond the heart
- PMID: 24007410
- PMCID: PMC3847505
- DOI: 10.1186/1475-2840-12-128
Mesenchymal stem cell transplantation for the infarcted heart: therapeutic potential for insulin resistance beyond the heart
Abstract
Background: This study aimed to evaluate the efficacy of mesenchymal stem cell (MSC) transplantation to mitigate abnormalities in cardiac-specific and systemic metabolism mediated by a combination of a myocardial infarction and diet-induced insulin resistance.
Methods: C57BL/6 mice were high-fat fed for eight weeks prior to induction of a myocardial infarction via chronic ligation of the left anterior descending coronary artery. MSCs were administered directly after myocardial infarction induction through a single intramyocardial injection. Echocardiography was performed prior to the myocardial infarction as well as seven and 28 days post-myocardial infarction. Hyperinsulinemic-euglycemic clamps coupled with 2-[14C]deoxyglucose were employed 36 days post-myocardial infarction (13 weeks of high-fat feeding) to assess systemic insulin sensitivity and insulin-mediated, tissue-specific glucose uptake in the conscious, unrestrained mouse. High-resolution respirometry was utilized to evaluate cardiac mitochondrial function in saponin-permeabilized cardiac fibers.
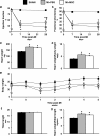
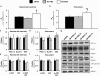
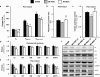
Results: MSC administration minimized the decline in ejection fraction following the myocardial infarction. The greater systolic function in MSC-treated mice was associated with increased in vivo cardiac glucose uptake and enhanced mitochondrial oxidative phosphorylation efficiency. MSC therapy promoted reductions in fasting arterial glucose and fatty acid concentrations. Additionally, glucose uptake in peripheral tissues including skeletal muscle and adipose tissue was elevated in MSC-treated mice. Enhanced glucose uptake in these tissues was associated with improved insulin signalling as assessed by Akt phosphorylation and prevention of a decline in GLUT4 often associated with high-fat feeding.
Conclusions: These studies provide insight into the utility of MSC transplantation as a metabolic therapy that extends beyond the heart exerting beneficial systemic effects on insulin action.
Figures

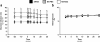
Similar articles
-
Diminishing impairments in glucose uptake, mitochondrial content, and ADP-stimulated oxygen flux by mesenchymal stem cell therapy in the infarcted heart.Am J Physiol Cell Physiol. 2014 Jan 1;306(1):C19-27. doi: 10.1152/ajpcell.00156.2013. Epub 2013 Nov 6. Am J Physiol Cell Physiol. 2014. PMID: 24196528 Free PMC article.
-
Mesenchymal stem cell transplantation for the infarcted heart: a role in minimizing abnormalities in cardiac-specific energy metabolism.Am J Physiol Endocrinol Metab. 2012 Jan 15;302(2):E163-72. doi: 10.1152/ajpendo.00443.2011. Epub 2011 Oct 4. Am J Physiol Endocrinol Metab. 2012. PMID: 21971524 Free PMC article.
-
Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression.J Thorac Cardiovasc Surg. 2010 Nov;140(5):1160-7. doi: 10.1016/j.jtcvs.2010.08.003. Epub 2010 Sep 17. J Thorac Cardiovasc Surg. 2010. PMID: 20850803
-
Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction.Cardiovasc Diabetol. 2019 Jan 9;18(1):1. doi: 10.1186/s12933-019-0806-4. Cardiovasc Diabetol. 2019. PMID: 30626440 Free PMC article.
-
Insulin regulation of myocardial autophagy.Circ J. 2014;78(11):2569-76. doi: 10.1253/circj.cj-14-1080. Epub 2014 Oct 20. Circ J. 2014. PMID: 25327953 Free PMC article. Review.
Cited by
-
Perspectives of induced pluripotent stem cells for cardiovascular system regeneration.Exp Biol Med (Maywood). 2015 May;240(5):549-56. doi: 10.1177/1535370214565976. Epub 2015 Jan 16. Exp Biol Med (Maywood). 2015. PMID: 25595188 Free PMC article. Review.
-
Neuroprotection of Human Umbilical Cord-Derived Mesenchymal Stem Cells (hUC-MSCs) in Alleviating Ischemic Stroke-Induced Brain Injury by Regulating Inflammation and Oxidative Stress.Neurochem Res. 2024 Oct;49(10):2871-2887. doi: 10.1007/s11064-024-04212-x. Epub 2024 Jul 18. Neurochem Res. 2024. PMID: 39026086
-
Addition of Prebiotics to the Ketogenic Diet Improves Metabolic Profile but Does Not Affect Seizures in a Rodent Model of Infantile Spasms Syndrome.Nutrients. 2022 May 26;14(11):2210. doi: 10.3390/nu14112210. Nutrients. 2022. PMID: 35684010 Free PMC article.
-
Mesenchymal stem cell therapy in type 2 diabetes mellitus.Diabetol Metab Syndr. 2017 May 15;9:36. doi: 10.1186/s13098-017-0233-1. eCollection 2017. Diabetol Metab Syndr. 2017. PMID: 28515792 Free PMC article. Review.
-
Glucose and fatty acid metabolism in infarcted heart from streptozotocin-induced diabetic rats after 2 weeks of tissue remodeling.Cardiovasc Diabetol. 2015 Nov 9;14:149. doi: 10.1186/s12933-015-0308-y. Cardiovasc Diabetol. 2015. PMID: 26553117 Free PMC article.
References
-
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79(1):215–262. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

