Mobile health (mHealth) based medication adherence measurement - a pilot trial using electronic blisters in diabetes patients
- PMID: 24007452
- PMCID: PMC3781679
- DOI: 10.1111/bcp.12184
Mobile health (mHealth) based medication adherence measurement - a pilot trial using electronic blisters in diabetes patients
Abstract
Aims: The aim of the present study was to evaluate a mobile health (mHealth) based remote medication adherence measurement system (mAMS) in elderly patients with increased cardiovascular risk treated for diabetes, high cholesterol and hypertension. Cardiovascular risk was defined as the presence of at least two out of the three risk factors: type 2 diabetes, hypercholesterolaemia and hypertension.
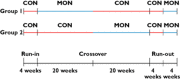
Methods: For treatment of diabetes, hypercholesterolaemia and hypertension, four predefined routinely used drugs were selected. Drug adherence was investigated in a controlled randomized doctor blinded study with crossover design. The mAMS was used to measure and improve objectively the adherence by means of closed-loop interactions.
Results: The mean age of the 53 patients (30 female) was 69.4 ± 4.8 years. A total of 1654 electronic blisters were handed out. A statistically significant difference (P = 0.04) between the monitoring and the control phase was observed for the diabetes medication only. In a post-study questionnaire twenty-nine patients appreciated that their physician knew if and when they had taken their medications and 13 asked for more or automated communication with their physicians. Only one subject withdrew from the study because of technical complexity.
Conclusions: The results indicate that mHealth based adherence management is feasible and well accepted by patients with increased cardiovascular risk. It may help to increase adherence, even in patients with high baseline adherence and, subsequently, lead to improved control of indicators including blood pressure and cholesterol concentrations. Electronic blisters can be used in a multi-medication regimen but need to be carefully designed for day-to-day application.
Keywords: adherence; diabetes; e-blister; mHealth; telemedicine.
© 2013 The British Pharmacological Society.
Figures



References
-
- Schülke AM, Plischke H, Kohls NB. Ambient Assistive Technologies (AAT): socio-technology as a powerful tool for facing the inevitable sociodemographic challenges? Philos Ethics Humanit Med. 2010;5:8. doi: 10.1186/1747-5341-5-8. - DOI - PMC - PubMed
-
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2•7 million participants. Lancet. 2011;378:31–40. - PubMed
-
- Hauner H, Köster I, von Ferber L. [Prevalence of diabetes mellitus in Germany 1998–2001. Secondary data analysis of a health insurance sample of the AOK in Hesse/KV in Hesse] Dtsch Med Wochenschr. 2003;128:2632–2637. - PubMed
-
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348:383–393. - PubMed
-
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

