A new approach to identify, classify and count drug-related events
- PMID: 24007453
- PMCID: PMC3781680
- DOI: 10.1111/bcp.12189
A new approach to identify, classify and count drug-related events
Abstract
Aims: The incidence of clinical events related to medication errors and/or adverse drug reactions reported in the literature varies by a degree that cannot solely be explained by the clinical setting, the varying scrutiny of investigators or varying definitions of drug-related events. Our hypothesis was that the individual complexity of many clinical cases may pose relevant limitations for current definitions and algorithms used to identify, classify and count adverse drug-related events.
Methods: Based on clinical cases derived from an observational study we identified and classified common clinical problems that cannot be adequately characterized by the currently used definitions and algorithms.
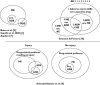
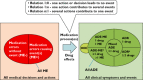
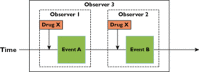
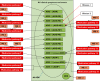
Results: It appears that some key models currently used to describe the relation of medication errors (MEs), adverse drug reactions (ADRs) and adverse drug events (ADEs) can easily be misinterpreted or contain logical inconsistencies that limit their accurate use to all but the simplest clinical cases. A key limitation of current models is the inability to deal with complex interactions such as one drug causing two clinically distinct side effects or multiple drugs contributing to a single clinical event. Using a large set of clinical cases we developed a revised model of the interdependence between MEs, ADEs and ADRs and extended current event definitions when multiple medications cause multiple types of problems. We propose algorithms that may help to improve the identification, classification and counting of drug-related events.
Conclusions: The new model may help to overcome some of the limitations that complex clinical cases pose to current paper- or software-based drug therapy safety.
Keywords: adverse drug event; adverse drug reaction; medication error; medication pathway; medication safety.
© 2013 The Authors. British Journal of Clinical Pharmacology © 2013 The British Pharmacological Society.
Figures

References
-
- Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002–2012. - PubMed
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. - PubMed
-
- Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10:199–205. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

