Evaluation of the hypersensitivity potential of alternative butter flavorings
- PMID: 24007741
- PMCID: PMC4683597
- DOI: 10.1016/j.fct.2013.08.053
Evaluation of the hypersensitivity potential of alternative butter flavorings
Abstract
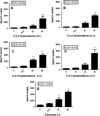
Concern has been raised over the association of diacetyl with lung disease clinically resembling bronchiolitis obliterans in food manufacturing workers. This has resulted in the need for identification of alternative chemicals to be used in the manufacturing process. Structurally similar chemicals, 2,3-pentanedione, 2,3-hexanedione, 3,4-hexanedione and 2,3-heptanedione, used as constituents of synthetic flavoring agents have been suggested as potential alternatives for diacetyl, however, immunotoxicity data on these chemicals are limited. The present study evaluated the dermal irritation and sensitization potential of diacetyl alternatives using a murine model. None of the chemicals were identified as dermal irritants when tested at concentrations up to 50%. Similar to diacetyl (EC3=17.9%), concentration-dependent increases in lymphocyte proliferation were observed following exposure to all four chemicals, with calculated EC3 values of 15.4% (2,3-pentanedione), 18.2% (2,3-hexanedione), 15.5% (3,4-hexanedione) and 14.1% (2,3-heptanedione). No biologically significant elevations in local or total serum IgE were identified after exposure to 25-50% concentrations of these chemicals. These results demonstrate the potential for development of hypersensitivity responses to these proposed alternative butter flavorings and raise concern about the use of structurally similar replacement chemicals. Additionally, a contaminant with strong sensitization potential was found in varying concentrations in diacetyl obtained from different producers.
Keywords: Butter flavorings; Diacetyl; Hypersensitivity; LLNA.
Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
-
- Akpinar-Elci M, Travis WD, Lynch DA, Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur. Respir. J. 2004;24:298–302. - PubMed
-
- Anderson SE, Wells JR, Fedorowicz A, Butterworth LF, Meade BJ, Munson AE. Evaluation of the contact and respiratory sensitization potential of volatile organic compounds generated by simulated indoor air chemistry. Toxicol. Sci. 2007;97:355–363. - PubMed
-
- Barrera M. No substitute for safety. Occup. Health Saf. 2011;80(32):34–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

