New insights into the pathology of podocyte loss: mitotic catastrophe
- PMID: 24007883
- PMCID: PMC3814687
- DOI: 10.1016/j.ajpath.2013.06.033
New insights into the pathology of podocyte loss: mitotic catastrophe
Abstract
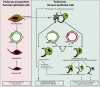
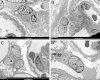
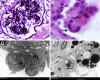
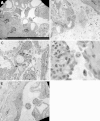
Podocytes represent an essential component of the kidney's glomerular filtration barrier. They stay attached to the glomerular basement membrane via integrin interactions that support the capillary wall to withstand the pulsating filtration pressure. Podocyte structure is maintained by a dynamic actin cytoskeleton. Terminal differentiation is coupled with permanent exit from the cell cycle and arrest in a postmitotic state. Postmitotic podocytes do not have an infinite life span; in fact, physiologic loss in the urine is documented. Proteinuria and other injuries accelerate podocyte loss or induce death. Mature podocytes are unable to replicate and maintain their actin cytoskeleton simultaneously. By the end of mitosis, cytoskeletal actin forms part of the contractile ring, rendering a round shape to podocytes. Therefore, when podocyte mitosis is attempted, it may lead to aberrant mitosis (ie, mitotic catastrophe). Mitotic catastrophe implies that mitotic podocytes eventually detach or die; this is a previously unrecognized form of podocyte loss and a compensatory mechanism for podocyte hypertrophy that relies on post-G1-phase cell cycle arrest. In contrast, local podocyte progenitors (parietal epithelial cells) exhibit a simple actin cytoskeleton structure and can easily undergo mitosis, supporting podocyte regeneration. In this review we provide an appraisal of the in situ pathology of mitotic catastrophe compared with other proposed types of podocyte death and put experimental and renal biopsy data in a unified perspective.
Copyright © 2013 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Kriz W., Shirato I., Nagata M., Lehir M., Lemley K.V. The podocyte’s response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304:F333–F347. - PubMed
-
- Wharram B.L., Goyal M., Wiggins J.E., Sanden S.K., Hussain S., Filipiak W.E., Saunders T.L., Dysko R.C., Kohno K., Holzman L.B., Wiggins R.C. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. - PubMed
-
- Mulay S.R., Thomasova D., Ryu M., Kulkarni O.P., Migliorini A., Bruns H., Gröbmayr R., Lazzeri E., Lasagni L., Liapis H., Romagnani P., Anders A.J. Podocyte loss involves MDM2-driven mitotic catastrophe. J Pathol. 2013;230:322–335. - PubMed
-
- Migliorini A., Angelotti M.L., Mulay S.R., Kulkarni O.O., Demleitner J., Dietrich A., Shankland S., Sagrinati C., Ballerini L., Peired A., Liapis H., Romagnani P., Anders H.J. The antiviral cytokines IFN-α and IFN-β modulate podocytes and parietal epithelial cells: implications for IFN toxicity, viral glomerulonephritis and glomerular regeneration. Am J Pathol. 2013;183:431–440. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

