Italian chemists' contributions to named reactions in organic synthesis: an historical perspective
- PMID: 24008246
- PMCID: PMC6270118
- DOI: 10.3390/molecules180910870
Italian chemists' contributions to named reactions in organic synthesis: an historical perspective
Abstract
From the second half of the 19th century up to modern times, the tremendous contribution of Italian chemists to the development of science resulted in the discovery of a number of innovative chemical transformations. These reactions were subsequently christened according to their inventors' name and so entered into the organic chemistry portfolio of "named organic reactions". As these discoveries were being conceived, massive social, political and geographical changes in these chemists' homeland were also occurring. In this review, a brief survey of known (and some lesser known) named organic reactions discovered by Italian chemists, along with their historical contextualization, is presented.
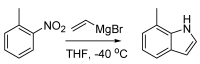
Figures
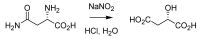
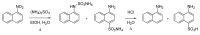






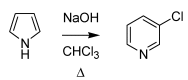





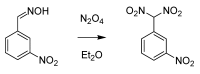



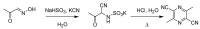
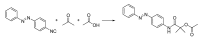
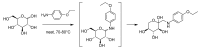
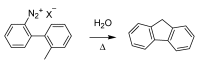















References
-
- Hassner A., Namboothiri I. Organic Synthesis Based on Name Reactions. 3rd ed. Elsevier; Amsterdam, The Netherlands: 2011.
-
- Li J.J. Name Reactions: A Collection of Detailed Reaction Mechanisms. 4th ed. Springer-Verlag; Berlin, Germany: 2009.
-
- Kürti L., Czakó B. Strategic Applications of Named Reactions in Organic Synthesis. Elsevier; Amsterdam, The Netherlands: 2005.
-
- Wang Z. Comprehensive Organic Name Reactions and Reagents. Wiley; Hoboken, NJ, USA: 2009.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

