Palmitoylation of estrogen receptors is essential for neuronal membrane signaling
- PMID: 24008343
- PMCID: PMC3800757
- DOI: 10.1210/en.2013-1172
Palmitoylation of estrogen receptors is essential for neuronal membrane signaling
Abstract
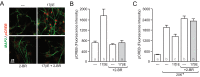
In addition to activating nuclear estrogen receptor signaling, 17β-estradiol can also regulate neuronal function via surface membrane receptors. In various brain regions, these actions are mediated by the direct association of estrogen receptors (ERs) activating metabotropic glutamate receptors (mGluRs). These ER/mGluR signaling partners are organized into discrete functional microdomains via caveolin proteins. A central question that remains concerns the underlying mechanism by which these subpopulations of ERs are targeted to the surface membrane. One candidate mechanism is S-palmitoylation, a posttranscriptional modification that affects the subcellular distribution and function of the modified protein, including promoting localization to membranes. Here we test for the role of palmitoylation and the necessity of specific palmitoylacyltransferase proteins in neuronal membrane ER action. In hippocampal neurons, pharmacological inhibition of palmitoylation eliminated 17β-estradiol-mediated phosphorylation of cAMP response element-binding protein, a process dependent on surface membrane ERs. In addition, mutation of the palmitoylation site on estrogen receptor (ER) α blocks ERα-mediated cAMP response element-binding protein phosphorylation. Similar results were obtained after mutation of the palmitoylation site on ERβ. Importantly, mutation of either ERα or ERβ did not affect the ability of the reciprocal ER to signal at the membrane. In contrast, membrane ERα and ERβ signaling were both dependent on the expression of the palmitoylacyltransferase proteins DHHC-7 and DHHC-21. Neither mGluR activity nor caveolin or ER expression was affected by knockdown of DHHC-7 and DHHC-21. These data collectively suggest discrete mechanisms that regulate specific isoform or global membrane ER signaling in neurons separate from mGluR activity or nuclear ER function.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

