Distinct cell shapes determine accurate chemotaxis
- PMID: 24008441
- PMCID: PMC3764443
- DOI: 10.1038/srep02606
Distinct cell shapes determine accurate chemotaxis
Abstract
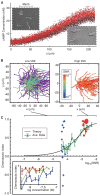
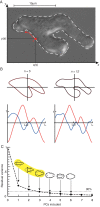
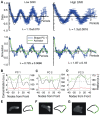
The behaviour of an organism often reflects a strategy for coping with its environment. Such behaviour in higher organisms can often be reduced to a few stereotyped modes of movement due to physiological limitations, but finding such modes in amoeboid cells is more difficult as they lack these constraints. Here, we examine cell shape and movement in starved Dictyostelium amoebae during migration toward a chemoattractant in a microfluidic chamber. We show that the incredible variety in amoeboid shape across a population can be reduced to a few modes of variation. Interestingly, cells use distinct modes depending on the applied chemical gradient, with specific cell shapes associated with shallow, difficult-to-sense gradients. Modelling and drug treatment reveals that these behaviours are intrinsically linked with accurate sensing at the physical limit. Since similar behaviours are observed in a diverse range of cell types, we propose that cell shape and behaviour are conserved traits.
Figures



References
-
- Hicheur H., Pham Q. C., Arechavaleta G., Laumond J. P. & Berthoz A. The formation of trajectories during goal-oriented locomotion in humans. I. A stereotyped behaviour. Eur. J. Neurosci. 26, 2376–2390 (2007). - PubMed
-
- Csermely D., Bonati B. & Romani R. Predatory behaviour of common kestrels (falco tinnunculus) in the wild. J. Ethol. 27, 461–465 (2009).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

