Resolving branched DNA intermediates with structure-specific nucleases during replication in eukaryotes
- PMID: 24008669
- PMCID: PMC3827899
- DOI: 10.1007/s00412-013-0431-z
Resolving branched DNA intermediates with structure-specific nucleases during replication in eukaryotes
Abstract
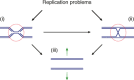
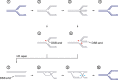
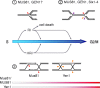
Genome duplication requires that replication forks track the entire length of every chromosome. When complications occur, homologous recombination-mediated repair supports replication fork movement and recovery. This leads to physical connections between the nascent sister chromatids in the form of Holliday junctions and other branched DNA intermediates. A key role in the removal of these recombination intermediates falls to structure-specific nucleases such as the Holliday junction resolvase RuvC in Escherichia coli. RuvC is also known to cut branched DNA intermediates that originate directly from blocked replication forks, targeting them for origin-independent replication restart. In eukaryotes, multiple structure-specific nucleases, including Mus81-Mms4/MUS81-EME1, Yen1/GEN1, and Slx1-Slx4/SLX1-SLX4 (FANCP) have been implicated in the resolution of branched DNA intermediates. It is becoming increasingly clear that, as a group, they reflect the dual function of RuvC in cleaving recombination intermediates and failing replication forks to assist the DNA replication process.
Figures

Similar articles
-
Replication intermediates that escape Dna2 activity are processed by Holliday junction resolvase Yen1.Nat Commun. 2016 Oct 25;7:13157. doi: 10.1038/ncomms13157. Nat Commun. 2016. PMID: 27779184 Free PMC article.
-
Functional overlap between the structure-specific nucleases Yen1 and Mus81-Mms4 for DNA-damage repair in S. cerevisiae.DNA Repair (Amst). 2010 Apr 4;9(4):394-402. doi: 10.1016/j.dnarep.2009.12.017. Epub 2010 Jan 27. DNA Repair (Amst). 2010. PMID: 20106725
-
Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes.Chromosoma. 2011 Apr;120(2):109-27. doi: 10.1007/s00412-010-0304-7. Epub 2011 Jan 11. Chromosoma. 2011. PMID: 21369956 Free PMC article. Review.
-
Distinct roles of Mus81, Yen1, Slx1-Slx4, and Rad1 nucleases in the repair of replication-born double-strand breaks by sister chromatid exchange.Mol Cell Biol. 2012 May;32(9):1592-603. doi: 10.1128/MCB.00111-12. Epub 2012 Feb 21. Mol Cell Biol. 2012. PMID: 22354996 Free PMC article.
-
Holliday junction resolution: regulation in space and time.DNA Repair (Amst). 2014 Jul;19(100):176-81. doi: 10.1016/j.dnarep.2014.03.013. Epub 2014 Apr 24. DNA Repair (Amst). 2014. PMID: 24767945 Free PMC article. Review.
Cited by
-
Heavy water inhibits DNA double-strand break repairs and disturbs cellular transcription, presumably via quantum-level mechanisms of kinetic isotope effects on hydrolytic enzyme reactions.PLoS One. 2024 Oct 3;19(10):e0309689. doi: 10.1371/journal.pone.0309689. eCollection 2024. PLoS One. 2024. PMID: 39361575 Free PMC article.
-
Collaboration in the actions of Brh2 with resolving functions during DNA repair and replication stress in Ustilago maydis.DNA Repair (Amst). 2018 Mar;63:47-55. doi: 10.1016/j.dnarep.2018.01.010. Epub 2018 Feb 2. DNA Repair (Amst). 2018. PMID: 29414053 Free PMC article.
-
Quantitative analysis of cell-free DNA in ovarian cancer.Oncol Lett. 2015 Dec;10(6):3478-3482. doi: 10.3892/ol.2015.3771. Epub 2015 Sep 30. Oncol Lett. 2015. PMID: 26788153 Free PMC article.
-
Replication fork reversal in eukaryotes: from dead end to dynamic response.Nat Rev Mol Cell Biol. 2015 Apr;16(4):207-20. doi: 10.1038/nrm3935. Epub 2015 Feb 25. Nat Rev Mol Cell Biol. 2015. PMID: 25714681 Review.
-
Limiting homologous recombination at stalled replication forks is essential for cell viability: DNA2 to the rescue.Curr Genet. 2020 Dec;66(6):1085-1092. doi: 10.1007/s00294-020-01106-7. Epub 2020 Sep 9. Curr Genet. 2020. PMID: 32909097 Free PMC article. Review.
References
-
- Aguilera A, Gómez-González B. Genome instability: a mechanistic view of its causes and consequences. Nat Rev Genet. 2008;9:204–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

