Neural tube defects--disorders of neurulation and related embryonic processes
- PMID: 24009034
- PMCID: PMC4023228
- DOI: 10.1002/wdev.71
Neural tube defects--disorders of neurulation and related embryonic processes
Abstract
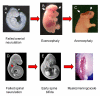
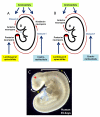
Neural tube defects (NTDs) are severe congenital malformations affecting 1 in every 1000 pregnancies. 'Open' NTDs result from failure of primary neurulation as seen in anencephaly, myelomeningocele (open spina bifida), and craniorachischisis. Degeneration of the persistently open neural tube in utero leads to loss of neurological function below the lesion level. 'Closed' NTDs are skin-covered disorders of spinal cord structure, ranging from asymptomatic spina bifida occulta to severe spinal cord tethering, and usually traceable to disruption of secondary neurulation. 'Herniation' NTDs are those in which meninges, with or without brain or spinal cord tissue, become exteriorized through a pathological opening in the skull or vertebral column (e.g., encephalocele and meningocele). NTDs have multifactorial etiology, with genes and environmental factors interacting to determine individual risk of malformation. While over 200 mutant genes cause open NTDs in mice, much less is known about the genetic causation of human NTDs. Recent evidence has implicated genes of the planar cell polarity signaling pathway in a proportion of cases. The embryonic development of NTDs is complex, with diverse cellular and molecular mechanisms operating at different levels of the body axis. Molecular regulatory events include the bone morphogenetic protein and Sonic hedgehog pathways which have been implicated in control of neural plate bending. Primary prevention of NTDs has been implemented clinically following the demonstration that folic acid (FA), when taken as a periconceptional supplement, can prevent many cases. Not all NTDs respond to FA, however, and adjunct therapies are required for prevention of this FA-resistant category.
Copyright © 2012 Wiley Periodicals, Inc.
Figures
Similar articles
-
Concepts in the neurosurgical care of patients with spinal neural tube defects: An embryologic approach.Birth Defects Res. 2019 Nov 15;111(19):1564-1576. doi: 10.1002/bdr2.1588. Epub 2019 Oct 2. Birth Defects Res. 2019. PMID: 31576681 Review.
-
Genetics and development of neural tube defects.J Pathol. 2010 Jan;220(2):217-30. doi: 10.1002/path.2643. J Pathol. 2010. PMID: 19918803 Free PMC article. Review.
-
Neural Tube Disorders(Archived).2023 Sep 15. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2023 Sep 15. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 32310363 Free Books & Documents.
-
Spina bifida and other neural tube defects.Curr Probl Pediatr. 2000 Nov-Dec;30(10):313-32. doi: 10.1067/mpp.2000.112052. Curr Probl Pediatr. 2000. PMID: 11147289 Review.
-
Mouse as a model for multifactorial inheritance of neural tube defects.Birth Defects Res C Embryo Today. 2012 Jun;96(2):193-205. doi: 10.1002/bdrc.21011. Birth Defects Res C Embryo Today. 2012. PMID: 22692891 Review.
Cited by
-
High glucose causes developmental abnormalities in neuroepithelial cysts with actin and HK1 distribution changes.Front Cell Dev Biol. 2023 Jan 6;10:1021284. doi: 10.3389/fcell.2022.1021284. eCollection 2022. Front Cell Dev Biol. 2023. PMID: 36684439 Free PMC article.
-
Intrinsic Mechanical Cues and Their Impact on Stem Cells and Embryogenesis.Front Cell Dev Biol. 2021 Nov 8;9:761871. doi: 10.3389/fcell.2021.761871. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34820380 Free PMC article. Review.
-
Premature Neural Progenitor Cell Differentiation Into Astrocytes in Retinoic Acid-Induced Spina Bifida Rat Model.Front Mol Neurosci. 2022 Jun 17;15:888351. doi: 10.3389/fnmol.2022.888351. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35782393 Free PMC article.
-
Prevalence of neural tube defects in England prior to the mandatory fortification of non-wholemeal wheat flour with folic acid: a population-based cohort study.Arch Dis Child. 2024 Jan 22;109(2):106-112. doi: 10.1136/archdischild-2023-325856. Arch Dis Child. 2024. PMID: 37875332 Free PMC article.
-
Single surgeon case series of myelomeningocele repairs in a developing world setting: Challenges and lessons.World Neurosurg X. 2023 May 19;19:100213. doi: 10.1016/j.wnsx.2023.100213. eCollection 2023 Jul. World Neurosurg X. 2023. PMID: 37260695 Free PMC article.
References
-
- Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Adv Exp Med Biol. 2010;686:349–364. - PubMed
-
- Mitchell LE. Epidemiology of neural tube defects. Am J Med Genet C Semin Med Genet. 2005;135:88–94. - PubMed
-
- Wood LR, Smith MT. Generation of anencephaly: 1. Aberrant neurulation and 2. Conversion of exencephaly to anencephaly. J Neuropath exp Neurol. 1984;43:620–633. - PubMed
-
- Seller MJ. Sex, neural tube defects, and multisite closure of the human neural tube. Am J Med Genet. 1995;58:332–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

