Functional interaction between amyloid-β precursor protein and peripherin neurofilaments: a shared pathway leading to Alzheimer's disease and amyotrophic lateral sclerosis?
- PMID: 24009040
- PMCID: PMC4286376
- DOI: 10.1159/000354238
Functional interaction between amyloid-β precursor protein and peripherin neurofilaments: a shared pathway leading to Alzheimer's disease and amyotrophic lateral sclerosis?
Abstract
Background and objective: The pathology of amyotrophic lateral sclerosis (ALS), a neurodegenerative disorder affecting motor neurons, comprises aberrant accumulations of neurofilaments; mutations in the peripherin subunit of neurofilaments have been identified in some forms of ALS. Recently, the amyloid-β precursor protein (APP), a key element for the pathology of Alzheimer's disease (AD), was linked to ALS. Here, we provide evidence that the generation of the N-terminal fragment of APP, sAPP, relies on peripherin neurofilaments. This finding could relate to a novel molecular mechanism dysregulated in ALS and/or AD.
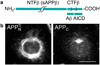
Methods and results: The production and the fate of sAPP were studied with the brainstem-derived, neuronal cell line, CAD, which expresses endogenous peripherin. We show that sAPP and C-terminal fragments (CTF) are generated to a large extent in the neuronal soma. We find that sAPP, but not CTF, associates with filamentous structures that delineate the nuclear lamina, extend to the cell periphery and immunostain for peripherin. The depletion of peripherin with siRNA eliminates the filamentous immunostaining of sAPP.
Conclusion: Our results indicate that a fraction of APP is cleaved by β-secretase in the soma and that the generated sAPP becomes associated with perinuclear peripherin neurofilaments. These findings link the metabolism of APP--which is dysregulated in AD--to the organization of neurofilaments--which is abnormal in ALS--and suggest a possible crosstalk/overlap between the molecular mechanisms of these diseases.
Copyright © 2013 S. Karger AG, Basel.
Figures


Similar articles
-
Shared Molecular Mechanisms in Alzheimer's Disease and Amyotrophic Lateral Sclerosis: Neurofilament-Dependent Transport of sAPP, FUS, TDP-43 and SOD1, with Endoplasmic Reticulum-Like Tubules.Neurodegener Dis. 2016;16(1-2):55-61. doi: 10.1159/000439256. Epub 2015 Nov 26. Neurodegener Dis. 2016. PMID: 26605911 Free PMC article.
-
Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain.J Neurochem. 1996 Nov;67(5):1882-96. doi: 10.1046/j.1471-4159.1996.67051882.x. J Neurochem. 1996. PMID: 8863493
-
Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β generation.Nat Commun. 2012 Apr 10;3:777. doi: 10.1038/ncomms1781. Nat Commun. 2012. PMID: 22491325 Free PMC article.
-
Impaired autophagy and APP processing in Alzheimer's disease: The potential role of Beclin 1 interactome.Prog Neurobiol. 2013 Jul-Aug;106-107:33-54. doi: 10.1016/j.pneurobio.2013.06.002. Epub 2013 Jul 1. Prog Neurobiol. 2013. PMID: 23827971 Review.
-
Amyloid precursor protein, copper and Alzheimer's disease.Biomed Pharmacother. 1997;51(3):105-11. doi: 10.1016/s0753-3322(97)86907-7. Biomed Pharmacother. 1997. PMID: 9181045 Review.
Cited by
-
Dual-tagged amyloid-β precursor protein reveals distinct transport pathways of its N- and C-terminal fragments.Hum Mol Genet. 2014 Mar 15;23(6):1631-43. doi: 10.1093/hmg/ddt555. Epub 2013 Nov 7. Hum Mol Genet. 2014. PMID: 24203698 Free PMC article.
-
Genetic compendium of 1511 human brains available through the UK Medical Research Council Brain Banks Network Resource.Genome Res. 2017 Jan;27(1):165-173. doi: 10.1101/gr.210609.116. Epub 2016 Dec 21. Genome Res. 2017. PMID: 28003435 Free PMC article.
-
Evidence for fungal infection in cerebrospinal fluid and brain tissue from patients with amyotrophic lateral sclerosis.Int J Biol Sci. 2015 Apr 2;11(5):546-58. doi: 10.7150/ijbs.11084. eCollection 2015. Int J Biol Sci. 2015. PMID: 25892962 Free PMC article.
-
ALS as a distal axonopathy: molecular mechanisms affecting neuromuscular junction stability in the presymptomatic stages of the disease.Front Neurosci. 2014 Aug 14;8:252. doi: 10.3389/fnins.2014.00252. eCollection 2014. Front Neurosci. 2014. PMID: 25177267 Free PMC article. Review.
-
Peripherin, A New Promising Biomarker in Neurological Disorders.Eur J Neurosci. 2025 Feb;61(4):e70030. doi: 10.1111/ejn.70030. Eur J Neurosci. 2025. PMID: 39995075 Free PMC article. Review.
References
-
- Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and ALS: A new look at an old question. Biochimica et Biophysica Acta. 2006;1762:1001–1012. - PubMed
-
- Bryson JB, Hobbs C, Parsons MJ, Bosch KD, Pandraud A, Walsh FS, Doherty P, Greensmith L. Amyloid precursor protein (APP) contributes to pathology in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Hum Mol Genet. 2012;21:3871–3882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

