Cysteine substitutions define etomidate binding and gating linkages in the α-M1 domain of γ-aminobutyric acid type A (GABAA) receptors
- PMID: 24009076
- PMCID: PMC3798502
- DOI: 10.1074/jbc.M113.494583
Cysteine substitutions define etomidate binding and gating linkages in the α-M1 domain of γ-aminobutyric acid type A (GABAA) receptors
Abstract
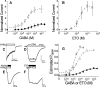
Etomidate is a potent general anesthetic that acts as an allosteric co-agonist at GABAA receptors. Photoreactive etomidate derivatives labeled αMet-236 in transmembrane domain M1, which structural models locate in the β+/α- subunit interface. Other nearby residues may also contribute to etomidate binding and/or transduction through rearrangement of the site. In human α1β2γ2L GABAA receptors, we applied the substituted cysteine accessibility method to α1-M1 domain residues extending from α1Gln-229 to α1Gln-242. We used electrophysiology to characterize each mutant's sensitivity to GABA and etomidate. We also measured rates of sulfhydryl modification by p-chloromercuribenzenesulfonate (pCMBS) with and without GABA and tested if etomidate blocks modification of pCMBS-accessible cysteines. Cys substitutions in the outer α1-M1 domain impaired GABA activation and variably affected etomidate sensitivity. In seven of eight residues where pCMBS modification was evident, rates of modification were accelerated by GABA co-application, indicating that channel activation increases water and/or pCMBS access. Etomidate reduced the rate of modification for cysteine substitutions at α1Met-236, α1Leu-232 and α1Thr-237. We infer that these residues, predicted to face β2-M3 or M2 domains, contribute to etomidate binding. Thus, etomidate interacts with a short segment of the outer α1-M1 helix within a subdomain that undergoes significant structural rearrangement during channel gating. Our results are consistent with in silico docking calculations in a homology model that orient the long axis of etomidate approximately orthogonal to the transmembrane axis.
Keywords: Allosteric Regulation; Anesthetics; GABA Receptors; Ion Channels; Nicotinic Acetylcholine Receptors; Sulfhydryl.
Figures





Similar articles
-
Tryptophan and Cysteine Mutations in M1 Helices of α1β3γ2L γ-Aminobutyric Acid Type A Receptors Indicate Distinct Intersubunit Sites for Four Intravenous Anesthetics and One Orphan Site.Anesthesiology. 2016 Dec;125(6):1144-1158. doi: 10.1097/ALN.0000000000001390. Anesthesiology. 2016. PMID: 27753644 Free PMC article.
-
Gating-induced conformational rearrangement of the γ-aminobutyric acid type A receptor β-α subunit interface in the membrane-spanning domain.J Biol Chem. 2012 Aug 10;287(33):27762-70. doi: 10.1074/jbc.M112.363341. Epub 2012 Jun 22. J Biol Chem. 2012. PMID: 22730325 Free PMC article.
-
Mutations at beta N265 in γ-aminobutyric acid type A receptors alter both binding affinity and efficacy of potent anesthetics.PLoS One. 2014 Oct 27;9(10):e111470. doi: 10.1371/journal.pone.0111470. eCollection 2014. PLoS One. 2014. PMID: 25347186 Free PMC article.
-
Anesthetic sites and allosteric mechanisms of action on Cys-loop ligand-gated ion channels.Can J Anaesth. 2011 Feb;58(2):191-205. doi: 10.1007/s12630-010-9419-9. Epub 2011 Jan 7. Can J Anaesth. 2011. PMID: 21213095 Free PMC article. Review.
-
GABA(A) receptors as molecular targets of general anesthetics: identification of binding sites provides clues to allosteric modulation.Can J Anaesth. 2011 Feb;58(2):206-15. doi: 10.1007/s12630-010-9429-7. Epub 2010 Dec 31. Can J Anaesth. 2011. PMID: 21194017 Free PMC article. Review.
Cited by
-
Substituted Cysteine Modification and Protection with n-Alkyl- Methanethiosulfonate Reagents Yields a Precise Estimate of the Distance between Etomidate and a Residue in Activated GABA Type A Receptors.Mol Pharmacol. 2021 Jun;99(6):426-434. doi: 10.1124/molpharm.120.000224. Epub 2021 Mar 25. Mol Pharmacol. 2021. PMID: 33766924 Free PMC article.
-
Tryptophan and Cysteine Mutations in M1 Helices of α1β3γ2L γ-Aminobutyric Acid Type A Receptors Indicate Distinct Intersubunit Sites for Four Intravenous Anesthetics and One Orphan Site.Anesthesiology. 2016 Dec;125(6):1144-1158. doi: 10.1097/ALN.0000000000001390. Anesthesiology. 2016. PMID: 27753644 Free PMC article.
-
Mapping General Anesthetic Sites in Heteromeric γ-Aminobutyric Acid Type A Receptors Reveals a Potential For Targeting Receptor Subtypes.Anesth Analg. 2016 Nov;123(5):1263-1273. doi: 10.1213/ANE.0000000000001368. Anesth Analg. 2016. PMID: 27167687 Free PMC article. Review.
-
Combining Mutations and Electrophysiology to Map Anesthetic Sites on Ligand-Gated Ion Channels.Methods Enzymol. 2018;602:369-389. doi: 10.1016/bs.mie.2018.01.014. Epub 2018 Feb 28. Methods Enzymol. 2018. PMID: 29588039 Free PMC article. Review.
-
The Direct Actions of GABA, 2'-Methoxy-6-Methylflavone and General Anaesthetics at β3γ2L GABAA Receptors: Evidence for Receptors with Different Subunit Stoichiometries.PLoS One. 2015 Oct 23;10(10):e0141359. doi: 10.1371/journal.pone.0141359. eCollection 2015. PLoS One. 2015. PMID: 26496640 Free PMC article.
References
-
- Franks N. P. (2008) General anaesthesia. From molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 9, 370–386 - PubMed
-
- Baumann S. W., Baur R., Sigel E. (2002) Forced subunit assembly in α1β2γ2 GABAA receptors. Insight into the absolute arrangement. J. Biol. Chem. 277, 46020–46025 - PubMed
-
- Jurd R., Arras M., Lambert S., Drexler B., Siegwart R., Crestani F., Zaugg M., Vogt K. E., Ledermann B., Antkowiak B., Rudolph U. (2003) General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 17, 250–252 - PubMed
-
- Reynolds D. S., Rosahl T. W., Cirone J., O'Meara G. F., Haythornthwaite A., Newman R. J., Myers J., Sur C., Howell O., Rutter A. R., Atack J., Macaulay A. J., Hadingham K. L., Hutson P. H., Belelli D., Lambert J. J., Dawson G. R., McKernan R., Whiting P. J., Wafford K. A. (2003) Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J. Neurosci. 23, 8608–8617 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

