The development of selective inhibitors of NagZ: increased susceptibility of Gram-negative bacteria to β-lactams
- PMID: 24009110
- PMCID: PMC3920638
- DOI: 10.1002/cbic.201300395
The development of selective inhibitors of NagZ: increased susceptibility of Gram-negative bacteria to β-lactams
Abstract
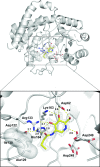
The increasing incidence of inducible chromosomal AmpC β-lactamases within the clinic is a growing concern because these enzymes deactivate a broad range of even the most recently developed β-lactam antibiotics. As a result, new strategies are needed to block the action of this antibiotic resistance enzyme. Presented here is a strategy to combat the action of inducible AmpC by inhibiting the β-glucosaminidase NagZ, which is an enzyme involved in regulating the induction of AmpC expression. A divergent route facilitating the rapid synthesis of a series of N-acyl analogues of 2-acetamido-2-deoxynojirimycin is reported here. Among these compounds are potent NagZ inhibitors that are selective against functionally related human enzymes. These compounds reduce minimum inhibitory concentration values for β-lactams against a clinically relevant Gram-negative bacterium bearing inducible chromosomal AmpC β-lactamase, Pseudomonas aeruginosa. The structure of a NagZ-inhibitor complex provides insight into the molecular basis for inhibition by these compounds.
Keywords: carbohydrates; enzymes; inhibitors; selectivity; synthesis.
© 2013 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA. This is an open access article under the terms of the Creative Commons Attribution License, which permits use, distribution and reproduction in any medium, provided the original work is properly cited.
Figures


Similar articles
-
Insight into a strategy for attenuating AmpC-mediated beta-lactam resistance: structural basis for selective inhibition of the glycoside hydrolase NagZ.Protein Sci. 2009 Jul;18(7):1541-51. doi: 10.1002/pro.137. Protein Sci. 2009. PMID: 19499593 Free PMC article.
-
Selective trihydroxyazepane NagZ inhibitors increase sensitivity of Pseudomonas aeruginosa to β-lactams.Chem Commun (Camb). 2013 Dec 4;49(93):10983-5. doi: 10.1039/c3cc46646a. Chem Commun (Camb). 2013. PMID: 24136176
-
Inactivation of the glycoside hydrolase NagZ attenuates antipseudomonal beta-lactam resistance in Pseudomonas aeruginosa.Antimicrob Agents Chemother. 2009 Jun;53(6):2274-82. doi: 10.1128/AAC.01617-08. Epub 2009 Mar 9. Antimicrob Agents Chemother. 2009. PMID: 19273679 Free PMC article.
-
[Progress in regulatory mechanism for inducing β-lactamase in Gram-negative bacteria].Sheng Wu Gong Cheng Xue Bao. 2018 Aug 25;34(8):1288-1296. doi: 10.13345/j.cjb.180187. Sheng Wu Gong Cheng Xue Bao. 2018. PMID: 30152214 Review. Chinese.
-
The sentinel role of peptidoglycan recycling in the β-lactam resistance of the Gram-negative Enterobacteriaceae and Pseudomonas aeruginosa.Bioorg Chem. 2014 Oct;56:41-8. doi: 10.1016/j.bioorg.2014.05.011. Epub 2014 Jun 4. Bioorg Chem. 2014. PMID: 24955547 Free PMC article. Review.
Cited by
-
Role of Enzymatic Activity in the Biological Cost Associated with the Production of AmpC β-Lactamases in Pseudomonas aeruginosa.Microbiol Spectr. 2022 Oct 26;10(5):e0270022. doi: 10.1128/spectrum.02700-22. Epub 2022 Oct 10. Microbiol Spectr. 2022. PMID: 36214681 Free PMC article.
-
DNA supercoiling and regulation of intrinsic β-lactamase in pathogenic Escherichia coli.Arch Microbiol. 2023 Nov 19;205(12):385. doi: 10.1007/s00203-023-03716-4. Arch Microbiol. 2023. PMID: 37980630 Review.
-
Cefazolin and imipenem enhance AmpC expression and resistance in NagZ-dependent manner in Enterobacter cloacae complex.BMC Microbiol. 2022 Nov 29;22(1):284. doi: 10.1186/s12866-022-02707-7. BMC Microbiol. 2022. PMID: 36443681 Free PMC article.
-
N-acetylglucosaminidases from CAZy family GH3 are really glycoside phosphorylases, thereby explaining their use of histidine as an acid/base catalyst in place of glutamic acid.J Biol Chem. 2015 Feb 20;290(8):4887-4895. doi: 10.1074/jbc.M114.621110. Epub 2014 Dec 22. J Biol Chem. 2015. PMID: 25533455 Free PMC article.
-
Conformational flexibility of the glycosidase NagZ allows it to bind structurally diverse inhibitors to suppress β-lactam antibiotic resistance.Protein Sci. 2017 Jun;26(6):1161-1170. doi: 10.1002/pro.3166. Epub 2017 Apr 7. Protein Sci. 2017. PMID: 28370529 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

