Structures and biogenetic analysis of lipofuscin bis-retinoids
- PMID: 24009196
- PMCID: PMC3773547
- DOI: 10.1631/jzus.B1300051
Structures and biogenetic analysis of lipofuscin bis-retinoids
Abstract
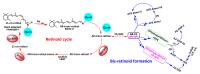
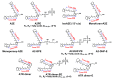
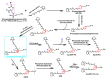
Age-related macular degeneration (AMD) is still an incurable blinding eye disease because of complex pathogenic mechanisms and unusual diseased regions. With the use of chemical biology tools, great progress has been achieved in improving the understanding of AMD pathogenesis. The severity of AMD is, at least in part, linked to the non-degradable lipofuscin bis-retinoids in retinal pigment epithelial (RPE). This material is thought to result from the lifelong accumulation of lysosomal residual bodies containing the end products derived from the daily phagocytosis of rod outer segments by RPE cells. Here, we present previously recognized bis-retinoids with focus on structures and biosynthetic pathways. In addition to a brief discussion on the mutual conversion relationships of bis-retinoids, future perspectives and the medical relevance of such studies on these lipofuscin constituents are also highlighted.
Conflict of interest statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Figures




Similar articles
-
Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: their origin is 11-cis-retinal.J Biol Chem. 2012 Jun 22;287(26):22276-86. doi: 10.1074/jbc.M111.329235. Epub 2012 May 8. J Biol Chem. 2012. PMID: 22570475 Free PMC article.
-
Inhibition of the ATP-driven proton pump in RPE lysosomes by the major lipofuscin fluorophore A2-E may contribute to the pathogenesis of age-related macular degeneration.FASEB J. 2004 Mar;18(3):562-4. doi: 10.1096/fj.03-0289fje. Epub 2004 Jan 8. FASEB J. 2004. PMID: 14715704
-
Experimental approaches to the study of A2E, a bisretinoid lipofuscin chromophore of retinal pigment epithelium.Methods Mol Biol. 2010;652:315-27. doi: 10.1007/978-1-60327-325-1_18. Methods Mol Biol. 2010. PMID: 20552437 Free PMC article.
-
The bisretinoids of retinal pigment epithelium.Prog Retin Eye Res. 2012 Mar;31(2):121-35. doi: 10.1016/j.preteyeres.2011.12.001. Epub 2011 Dec 22. Prog Retin Eye Res. 2012. PMID: 22209824 Free PMC article. Review.
-
Bisretinoids of RPE lipofuscin: trigger for complement activation in age-related macular degeneration.Adv Exp Med Biol. 2010;703:63-74. doi: 10.1007/978-1-4419-5635-4_5. Adv Exp Med Biol. 2010. PMID: 20711707 Review.
Cited by
-
Continuous Flow Synthesis of A2E Guided by Design of Experiments and High-Throughput Studies.ACS Bio Med Chem Au. 2022 Feb 18;2(3):297-306. doi: 10.1021/acsbiomedchemau.1c00060. eCollection 2022 Jun 15. ACS Bio Med Chem Au. 2022. PMID: 37101569 Free PMC article.
-
All-trans-retinoic acid generation is an antidotal clearance pathway for all-trans-retinal in the retina.J Zhejiang Univ Sci B. 2019 Dec.;20(12):960-971. doi: 10.1631/jzus.B1900271. J Zhejiang Univ Sci B. 2019. PMID: 31749343 Free PMC article.
-
Clinical applications of retinal gene therapies.Precis Clin Med. 2018 Jun;1(1):5-20. doi: 10.1093/pcmedi/pby004. Epub 2018 Jun 1. Precis Clin Med. 2018. PMID: 35694125 Free PMC article. Review.
-
Bis(monoacylglycero)phosphate lipids in the retinal pigment epithelium implicate lysosomal/endosomal dysfunction in a model of Stargardt disease and human retinas.Sci Rep. 2017 Dec 11;7(1):17352. doi: 10.1038/s41598-017-17402-1. Sci Rep. 2017. PMID: 29229934 Free PMC article.
-
Effects of organic solvents on two retinal pigment epithelial lipofuscin fluorophores, A2E and all-trans-retinal dimer.J Zhejiang Univ Sci B. 2014 Jul;15(7):661-9. doi: 10.1631/jzus.B1300194. J Zhejiang Univ Sci B. 2014. PMID: 25001225 Free PMC article.
References
-
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, et al. A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet. 1997;15(3):236–246. doi: 10.1038/ng0397-236. - DOI - PubMed
-
- Ben-Shabat S, Itagaki Y, Jockusch S, Sparrow JR, Turro NJ, Nakanishi K. Formation of a nonaoxirane from A2E, a lipofuscin fluorophore related to macular degeneration, and evidence of singlet oxygen involvement. Angew Chem Int Ed. 2002;41(5):814–817. doi: 10.1002/1521-3773(20020301)41:5<814::AID-ANIE814>3.0.CO;2-2. - DOI - PubMed
-
- Birnbach CD, Jarvelainen M, Possin DE, Milam AH. Histopathology and immunocytochemistry of the neurosensory retina in fundus flavimaculatus. Ophthalmology. 1994;101(7):1211–1219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

