Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD
- PMID: 24009239
- PMCID: PMC3871767
- DOI: 10.1681/ASN.2012090962
Cysteamine modulates oxidative stress and blocks myofibroblast activity in CKD
Abstract
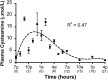
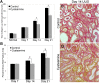
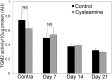
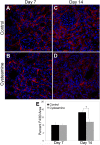
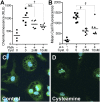
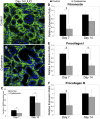
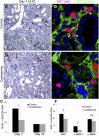
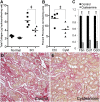
Therapy to slow the relentless expansion of interstitial extracellular matrix that leads to renal functional decline in patients with CKD is currently lacking. Because chronic kidney injury increases tissue oxidative stress, we evaluated the antifibrotic efficacy of cysteamine bitartrate, an antioxidant therapy for patients with nephropathic cystinosis, in a mouse model of unilateral ureteral obstruction. Fresh cysteamine (600 mg/kg) was added to drinking water daily beginning on the day of surgery, and outcomes were assessed on days 7, 14, and 21 after surgery. Plasma cysteamine levels showed diurnal variation, with peak levels similar to those observed in patients with cystinosis. In cysteamine-treated mice, fibrosis severity decreased significantly at 14 and 21 days after unilateral ureteral obstruction, and renal oxidized protein levels decreased at each time point, suggesting reduced oxidative stress. Consistent with these results, treatment of cultured macrophages with cysteamine reduced cellular generation of reactive oxygen species. Furthermore, treatment with cysteamine reduced α-smooth muscle actin-positive interstitial myofibroblast proliferation and mRNA levels of extracellular matrix proteins in mice and attenuated myofibroblast differentiation and proliferation in vitro, but did not augment TGF-β signaling. In a study of renal ischemia reperfusion, cysteamine therapy initiated 10 days after injury and continued for 14 days decreased renal fibrosis by 40%. Taken together, these data suggest previously unrecognized antifibrotic actions of cysteamine via TGF-β-independent mechanisms that include oxidative stress reduction and attenuation of the myofibroblast response to kidney injury and support further investigation into the potential benefit of cysteamine therapy in the treatment of CKD.
Figures



Comment in
-
Antifibrotic therapy: is an antioxidative regimen the answer?J Am Soc Nephrol. 2014 Jan;25(1):3-5. doi: 10.1681/ASN.2013101034. Epub 2013 Oct 31. J Am Soc Nephrol. 2014. PMID: 24179167 Free PMC article. No abstract available.
References
-
- Okamura DM, Himmelfarb J: Tipping the redox balance of oxidative stress in fibrogenic pathways in chronic kidney disease. Pediatr Nephrol 24: 2309–2319, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

