Enzalutamide as a second generation antiandrogen for treatment of advanced prostate cancer
- PMID: 24009414
- PMCID: PMC3762760
- DOI: 10.2147/DDDT.S45703
Enzalutamide as a second generation antiandrogen for treatment of advanced prostate cancer
Abstract
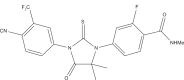
Prostate cancer (PCa) is the most common malignancy, and the third leading cancer-related cause of death among men of the Western world. Upon PCa progression into metastatic disease, androgen deprivation therapy is applied as the first-line treatment, and has been shown to be effective in most patients, leading to a decrease in serum prostate-specific antigen and relief of disease-related symptoms. However, advanced PCa almost inevitably progresses to a castration-resistant state, and is currently regarded as incurable. The large body of evidence indicates that PCa cells remain dependent on androgen receptor (AR) signaling even in an androgen-deprived environment. As such, development of drugs that target AR and AR signaling pathways have become one of the major milestones in treatment of castration-resistant PCa (CRPC). Nevertheless, currently available therapies that target AR signaling are still regarded as palliative and more potent therapies are in great need. Over the past few years, a wide range of novel therapies has entered clinical trial for treatment of CRPC, including androgen synthesis inhibitors (abiraterone acetate), chemotherapeutic agents (docetaxel and cabazitaxel), and immunotherapies (sipuleucel-T). In this context, enzalutamide (previously referred to as MDV3100) is a novel second generation antiandrogen that has been demonstrated to significantly improve survival in men with metastatic CRPC in several clinical trials. In this paper we summarize recently completed and ongoing clinical trials of enzalutamide, and briefly discuss the efficacy of the novel antiandrogen therapy and its limitations for treatment of CRPC.
Keywords: castration resistant prostate cancer; clinical trials; drug resistance.
Figures

References
-
- GLOBOCAN v1.2 Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. 2008.
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62(1):10–29. - PubMed
-
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;167(2 Pt 2):948–951. discussion 952. - PubMed
-
- Huggins C. The hormone-dependent cancers. JAMA. 1963;186:481–483. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

