Structural similarities and differences between amyloidogenic and non-amyloidogenic islet amyloid polypeptide (IAPP) sequences and implications for the dual physiological and pathological activities of these peptides
- PMID: 24009497
- PMCID: PMC3757079
- DOI: 10.1371/journal.pcbi.1003211
Structural similarities and differences between amyloidogenic and non-amyloidogenic islet amyloid polypeptide (IAPP) sequences and implications for the dual physiological and pathological activities of these peptides
Abstract
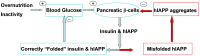
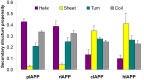
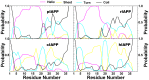
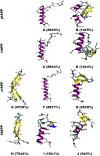
IAPP, a 37 amino-acid peptide hormone belonging to the calcitonin family, is an intrinsically disordered protein that is coexpressed and cosecreted along with insulin by pancreatic islet β-cells in response to meals. IAPP plays a physiological role in glucose regulation; however, in certain species, IAPP can aggregate and this process is linked to β-cell death and Type II Diabetes. Using replica exchange molecular dynamics with extensive sampling (16 replicas per sequence and 600 ns per replica), we investigate the structure of the monomeric state of two species of aggregating peptides (human and cat IAPP) and two species of non-aggregating peptides (pig and rat IAPP). Our simulations reveal that the pig and rat conformations are very similar, and consist of helix-coil and helix-hairpin conformations. The aggregating sequences, on the other hand, populate the same helix-coil and helix-hairpin conformations as the non-aggregating sequence, but, in addition, populate a hairpin structure. Our exhaustive simulations, coupled with available peptide-activity data, leads us to a structure-activity relationship (SAR) in which we propose that the functional role of IAPP is carried out by the helix-coil conformation, a structure common to both aggregating and non-aggregating species. The pathological role of this peptide may have multiple origins, including the interaction of the helical elements with membranes. Nonetheless, our simulations suggest that the hairpin structure, only observed in the aggregating species, might be linked to the pathological role of this peptide, either as a direct precursor to amyloid fibrils, or as part of a cylindrin type of toxic oligomer. We further propose that the helix-hairpin fold is also a possible aggregation prone conformation that would lead normally non-aggregating variants of IAPP to form fibrils under conditions where an external perturbation is applied. The SAR relationship is used to suggest the rational design of therapeutics for treating diabetes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Distinct helix propensities and membrane interactions of human and rat IAPP(1-19) monomers in anionic lipid bilayers.J Phys Chem B. 2015 Feb 26;119(8):3366-76. doi: 10.1021/jp5111357. Epub 2015 Feb 17. J Phys Chem B. 2015. PMID: 25646717
-
Membrane disruption and early events in the aggregation of the diabetes related peptide IAPP from a molecular perspective.Acc Chem Res. 2012 Mar 20;45(3):454-62. doi: 10.1021/ar200189b. Epub 2011 Sep 25. Acc Chem Res. 2012. PMID: 21942864 Free PMC article.
-
Comparing the structural properties of human and rat islet amyloid polypeptide by MD computer simulations.Biophys Chem. 2011 Jun;156(1):43-50. doi: 10.1016/j.bpc.2010.12.007. Epub 2010 Dec 31. Biophys Chem. 2011. PMID: 21266296
-
Islet amyloid and type 2 diabetes: from molecular misfolding to islet pathophysiology.Biochim Biophys Acta. 2001 Nov 29;1537(3):179-203. doi: 10.1016/s0925-4439(01)00078-3. Biochim Biophys Acta. 2001. PMID: 11731221 Review.
-
Aggregation of islet amyloid polypeptide: from physical chemistry to cell biology.Curr Opin Struct Biol. 2013 Feb;23(1):82-9. doi: 10.1016/j.sbi.2012.11.003. Epub 2012 Dec 22. Curr Opin Struct Biol. 2013. PMID: 23266002 Free PMC article. Review.
Cited by
-
β-Hairpin of Islet Amyloid Polypeptide Bound to an Aggregation Inhibitor.Sci Rep. 2016 Sep 19;6:33474. doi: 10.1038/srep33474. Sci Rep. 2016. PMID: 27641459 Free PMC article.
-
Graphene quantum dots against human IAPP aggregation and toxicity in vivo.Nanoscale. 2018 Nov 1;10(42):19995-20006. doi: 10.1039/c8nr07180b. Nanoscale. 2018. PMID: 30350837 Free PMC article.
-
Can Alzheimer's Disease Be Secondary to Type-2 Diabetes Mellitus?Cureus. 2022 Nov 8;14(11):e31273. doi: 10.7759/cureus.31273. eCollection 2022 Nov. Cureus. 2022. PMID: 36505102 Free PMC article. Review.
-
Protein misfolding and aggregation in Alzheimer's disease and type 2 diabetes mellitus.CNS Neurol Disord Drug Targets. 2014;13(7):1280-93. doi: 10.2174/1871527313666140917095514. CNS Neurol Disord Drug Targets. 2014. PMID: 25230234 Free PMC article.
-
In silico studies of the human IAPP in the presence of osmolytes.J Mol Model. 2022 Jun 14;28(7):188. doi: 10.1007/s00894-022-05180-1. J Mol Model. 2022. PMID: 35697975
References
-
- Pittner RA, Albrandt K, Beaumont K, Gaeta LSL, Koda JE, et al. (1994) Molecular Physiology of Amylin. J Cell Biochem 55: 19–28. - PubMed
-
- Young AA, Vine W, Gedulin BR, Pittner R, Janes S, et al. (1996) Preclinical pharmacology of pramlintide in the rat: Comparisons with human and rat amylin. Drug Dev Res 37: 231–248.
-
- Kruger DF, Gatcomb PM, Owen SK (1999) Clinical implications of amylin and amylin deficiency. Diabetes Educ 25: 389–397. - PubMed
-
- Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, et al. (2004) Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med 21: 1204–1212. - PubMed
-
- Cooper GJS, Day AJ, Willis AC, Roberts AN, Reid KBM, et al. (1989) Amylin and the Amylin Gene - Structure, Function and Relationship to Islet Amyloid and to Diabetes-Mellitus. Biochim Biophys Acta 1014: 247–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

